Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteoarthritis (OA) is rising at an alarming rate, affecting millions worldwide and threatening mobility, independence, and quality of life. High-quality evidence is essential to guide clinical decision-making and ensure the best patient outcomes. The discontinuation and non-publication of clinical studies are major contributors to research waste and incomplete data synthesis in evidence-based medicine. This study aims to assess the prevalence, characteristics, and publication history of OA clinical studies and investigate the causes for their non-publication or discontinuation.
Methods: We searched OA-related clinical trials registered in ClinicalTrials.gov using NCT identifiers up to January 2nd, 2025. We excluded the last 24 months to account for ongoing peer reviews. Studies were considered discontinued if terminated, withdrawn, or suspended and unpublished if their results were not available in a peer-reviewed journal. Data on gender, age, study type, funding, intervention, enrollment, and location were extracted. Multiple logistic regression analyzed characteristics linked to unpublished or discontinued trials. All statistical analyses were performed using Jamovi 2.6.24 software.
Results: A total of 3,818 studies were included, comprising 2,706 (70.9%) completed, 425 (11.1%) discontinued, and 687 (17.9%) with unknown status (Fig. 1). Of the completed and discontinued studies, 2,588 were interventional and 538 were observational. Among the 425 discontinued studies, 9.4% of them were published despite being discontinued, and 34.8% of unknown studies were also found published. Also, nearly half (43.3%) of completed studies remained unpublished (table 1). Logistic regression analysis revealed that non-industry-funded studies had lower odds of discontinuation than industry-funded ones (OR = 0.380, 95% CI: 0.279–0.518, p < 0.001). Device-based interventions were nearly three times more likely to be discontinued than behavioral interventions (OR = 2.977, 95% CI: 1.445–6.133, p = 0.003). Trials with >100 participants had lower odds of discontinuation than those < 100 (OR = 0.371, 95% CI 0.278, 0.495, p < 0.001). As for publication, behavioral interventions were significantly more likely to be published compared to device intervention (OR = 2.346, 95% CI: 1.594 to 3.451, p < 0.001), enrollment sizes >100 were more likely to be published than those < 100 (OR = 0.575, 95% CI: 0.479 to 0.690), and non-industry-funded studies were more likely to be published than industry-funded ones (OR = 0.409, 95% CI: 0.326 to 0.513). Notably, the United States was the most common location for conducting OA clinical trials 1,072 (28.4%), and also had the highest discontinuation rate globally (39.2%) (Fig. 2).
Conclusion: OA clinical trials are frequently discontinued and often remain unpublished, restricting access to valuable research findings. This lack of transparency not only hinders scientific progress but also raises ethical concerns about participant contributions. Ensuring transparency through mandatory reporting and stricter policies can help address publication bias and ensure that patients efforts contribute meaningfully to medical knowledge.
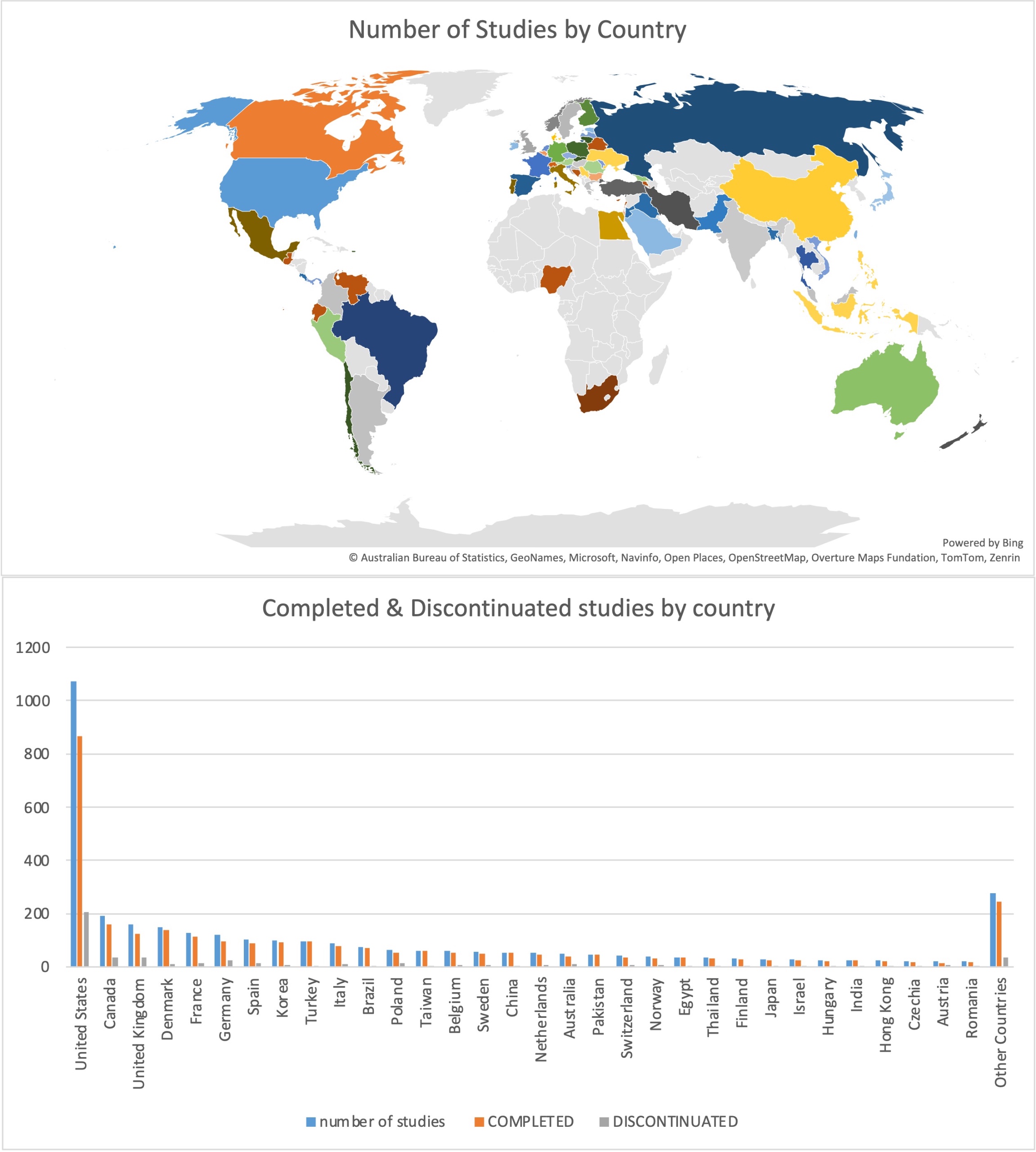 Figure (1) PRISMA Flow Chart Illustrating Search Strategy and Selection of Included Studies
Figure (1) PRISMA Flow Chart Illustrating Search Strategy and Selection of Included Studies
.jpg) (Figure 2) Global Distribution of Total, Completed and Discontinued clinicals studies by Country.
(Figure 2) Global Distribution of Total, Completed and Discontinued clinicals studies by Country.
.jpg) (Table 1) Characteristics and outcomes of OA clinical Studies by completed, discontinued, published and nonpublished status
(Table 1) Characteristics and outcomes of OA clinical Studies by completed, discontinued, published and nonpublished status
To cite this abstract in AMA style:
Abdelsalam M, Hafez H, Lasheen M, Badwy B, El Sedafy O, Hafez A, El-Sayed O, Mahdy M, Hassanin A, Awad M, Hamza M. Discontinuation and Non-Publication of Osteoarthritis Clinical Studies: A Cross-Sectional Analysis of 10,686,413 Patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/discontinuation-and-non-publication-of-osteoarthritis-clinical-studies-a-cross-sectional-analysis-of-10686413-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-and-non-publication-of-osteoarthritis-clinical-studies-a-cross-sectional-analysis-of-10686413-patients/
