Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Basket trials group together participants who share a common biomarker or disease pathway across several diseases or phenotypes in a single clinical study. Such trial designs may substantially increase efficiency of clinical development, especially in rare diseases with the challenges of limited resources and high operation costs. Rituximab, a monoclonal antibody targeting the CD20 antigen on B-cells, is used as a treatment option for several rare autoimmune diseases including autoimmune hemolytic anemia (AIHA), thrombotic thrombocytopenic purpura (TTP), and idiopathic thrombocytopenic purpura (ITP), as it effectively depletes B-cells which are often implicated in the production of autoantibodies causing these conditions.
Methods: We utilized real world data (RWD) of off-label use of rituximab together with literature data for a design of a randomized confirmatory basket trial of steroid therapy +/- rituximab in multiple rare auto-immune diseases. The adaptive basket design removes disease indications where the therapy does not appear to be benefitting patients at interim analysis, pools the remaining indications in the primary analysis, and then performs an indication-by-indication post check, again discarding indications that do not appear to benefit patients (Figure 1). We simulated this basket design under numerous scenarios varying design parameters based on RWD and literature information and assessed power, efficiency, and benefit of a basket trial investigating k indications compared to k parallel, independent Phase 3 designs, given the false positive rate of the entire trial controlled at 0.025k. Efficiency was defined as the number of true positive results confirmed per patient enrolled. A false positive event was considered to have occurred if the study resulted in approval of one or more indications where experimental therapy was not beneficial.
Results: Information from RWD influenced our selection of indications for the simulated trial and led us to increase the sample size due to more realistic estimates of clinical benefit. Literature information alone resulted in poor study performance. Using the RWD information together with literature information, we were able to design a basket trial of three auto-immune indications that had robust performance across plausible clinical scenarios, and up to a 46-132% improvement in development efficiency with comparable control of the false positive rate when compared to parallel clinical studies.
Conclusion: RWD can be useful when performing design optimization of complex basket trial designs in rare auto-immune disease. Rigorous, efficient confirmatory basket trial designs are feasible.
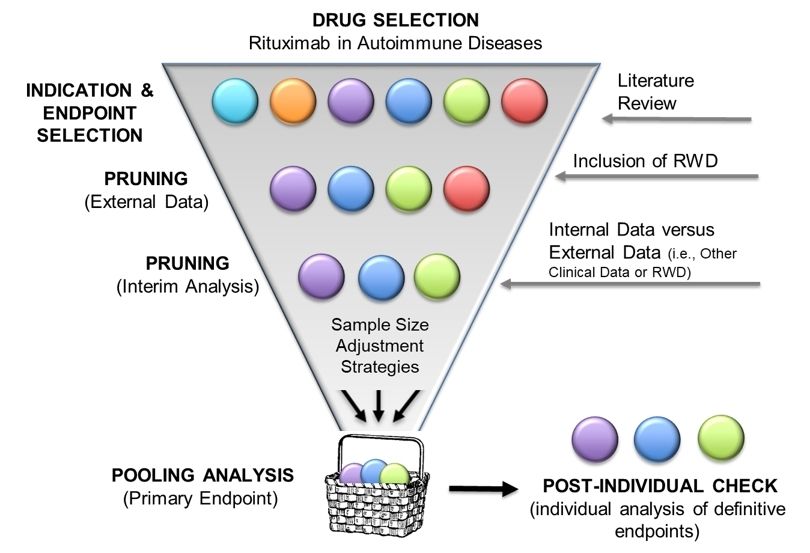 Figure 1. Randomized confirmatory basket designs. Cartoon shapes represent putative indications. The design is a funnel proceeding from top to bottom with the indicated steps shown on the side of the funnel. After indication and endpoint selection by literature review, RWD is used to optimize the choice of indications and other detailed study design features. A pruning process subjects possible indications to a high interim bar to decrease heterogeneity. After this pruning, the resulting pooled indications continue until study maturity. A pooled analysis on pooled data by the definitive endpoint is applied and followed by an additional pruning step to individual indications if the pooled analysis is positive, which enables stricter control of the false positive rate by individual indications.
Figure 1. Randomized confirmatory basket designs. Cartoon shapes represent putative indications. The design is a funnel proceeding from top to bottom with the indicated steps shown on the side of the funnel. After indication and endpoint selection by literature review, RWD is used to optimize the choice of indications and other detailed study design features. A pruning process subjects possible indications to a high interim bar to decrease heterogeneity. After this pruning, the resulting pooled indications continue until study maturity. A pooled analysis on pooled data by the definitive endpoint is applied and followed by an additional pruning step to individual indications if the pooled analysis is positive, which enables stricter control of the false positive rate by individual indications.
To cite this abstract in AMA style:
Guinn D, He L, Ren Y, Korostyshevskiy V, Beckman R. Randomized Confirmatory Basket Trial: Performance Evaluation of a Simulated Application Example in Rare Disease Using Real World Data [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/randomized-confirmatory-basket-trial-performance-evaluation-of-a-simulated-application-example-in-rare-disease-using-real-world-data/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/randomized-confirmatory-basket-trial-performance-evaluation-of-a-simulated-application-example-in-rare-disease-using-real-world-data/
