Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Anifrolumab is a human monoclonal antibody targeting the type I interferon receptor, approved for the treatment of systemic lupus erythematosus (SLE), with demonstrated efficacy in managing cutaneous involvement. However, its potential role in non-systemic subacute and chronic cutaneous lupus remains undefined, as its use in this setting is off-label.This study aimed to assess the effectiveness and safety of anifrolumab in patients with non-systemic subacute or chronic cutaneous lupus in routine clinical practice.
Methods: This multicenter study evaluated patients with non-systemic subacute or chronic cutaneous lupus treated with anifrolumab. Given that non-systemic lupus represents an off-label indication, informed consent was obtained from all participants.Cutaneous involvement was assessed using the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI), evaluating both disease activity (CLASI-A, score range: 0–70) and damage (CLASI-D, score range: 0–56). The primary outcome was the reduction in CLASI-A and CLASI-D scores. Comparisons were conducted between baseline and months 1, 3, and 6 using Wilcoxon’s signed-rank test.Furthermore, considering the role of natural killer (NK) cells in the pathogenesis of cutaneous lupus, we analyzed NK cell dynamics in three patients over the study period.
Results: A total of 10 patients (8 females, 2 males) with cutaneous lupus treated with anifrolumab were included in the analysis (TABLE). The diagnoses were discoid lupus (n=7) and subacute lupus (n=3). The mean disease duration prior to anifrolumab initiation was 32.9±30.2 months.Before starting anifrolumab, patients had received the following treatments: topical glucocorticoids (n=10), hydroxychloroquine (n=10), methotrexate (n=7), oral glucocorticoids (n=5), chloroquine (n=3), azathioprine (n=2), belimumab (n=2), rituximab (n=2), mepacrine (n=1), thalidomide (n=1), apremilast (n=1), and mycophenolate mofetil (n=1).Anifrolumab was administered at a dose of 300 mg intravenously every 4 weeks, either in combination with other therapies (n=9) or as monotherapy (n=1). All patients experienced a rapid and significant improvement in cutaneous disease activity (CLASI-A and CLASI-D) following anifrolumab initiation (FIGURE 1).During a mean follow-up of 5.3±4.6 months, the median [IQR] CLASI-A score decreased from 16.5 [12.7–21.5] to 1 [1–3.2] (p=0.032), while the median [IQR] CLASI-D score decreased from 8 [4.2–9.7] to 4 [2.5–6.7] (p=0.044). No adverse effects were reported during follow-up. Additionally, an upregulation trend in inhibitory markers was observed in NK cell subtypes among the three patients in whom this analysis was performed (FIGURE 2).
Conclusion: Anifrolumab appears to be an effective and well-tolerated treatment for patients with refractory non-systemic cutaneous lupus.
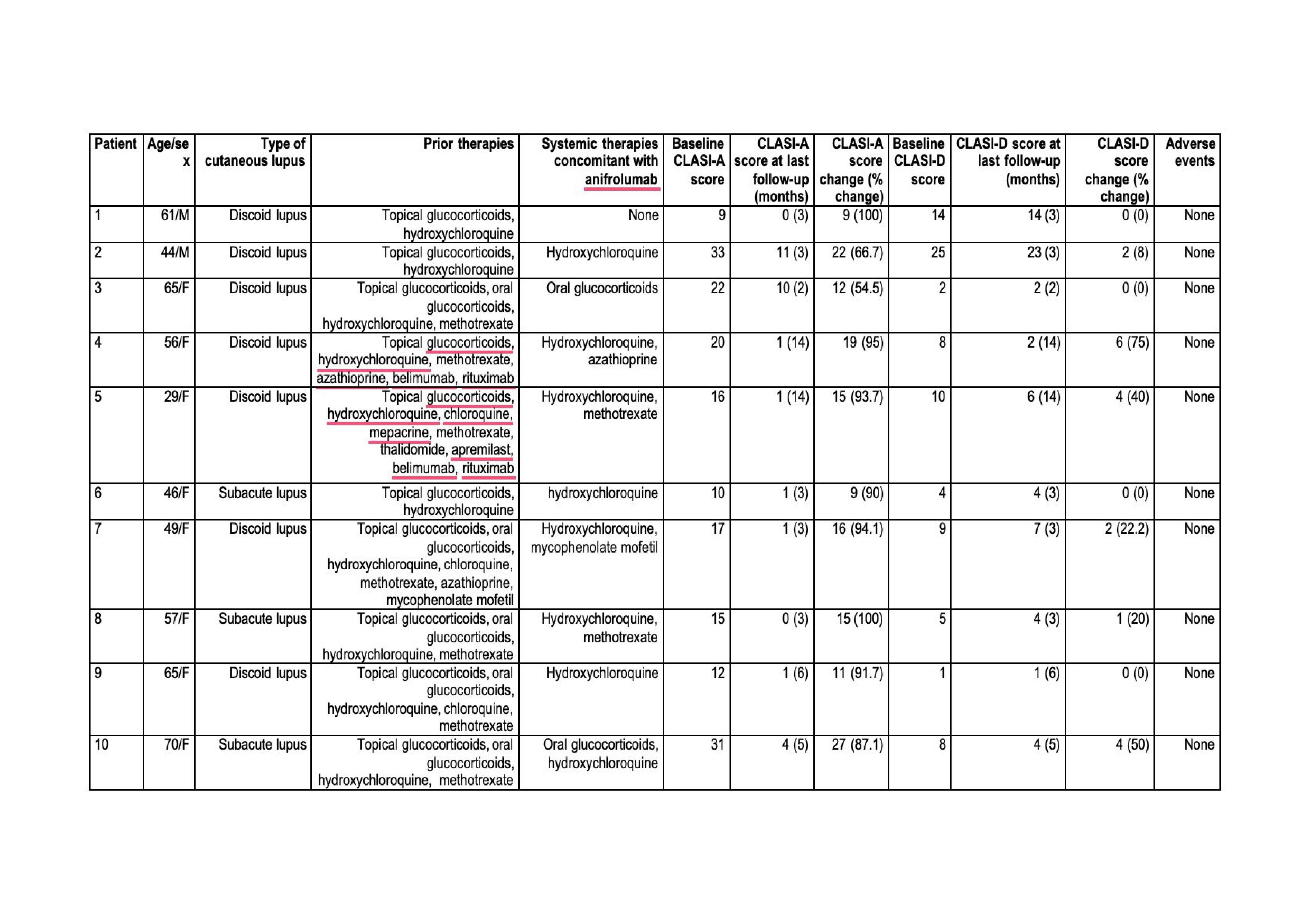 TABLE. Main features of the 8 patients with cutaneous lupus treated with anifrolumab.
TABLE. Main features of the 8 patients with cutaneous lupus treated with anifrolumab.
.jpg) FIGURE 1. Clinical improvement of cutaneous lupus in patients treated with anifrolumab.
FIGURE 1. Clinical improvement of cutaneous lupus in patients treated with anifrolumab.
To cite this abstract in AMA style:
Lasa Teja C, Loricera J, López-Hoyos M, Bejerano-Herreria C, Estébanez A, Sanmartin Martínez M, González-Fernández M, Ferraz Amaro I, Blanco R. Effectiveness and safety of Anifrolumab in Non-Systemic Cutaneous Lupus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-anifrolumab-in-non-systemic-cutaneous-lupus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-anifrolumab-in-non-systemic-cutaneous-lupus/

.jpg)