Session Information
Date: Sunday, October 26, 2025
Title: (0210–0232) Measures & Measurement of Healthcare Quality Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Non-oncologic use of immunomodulatory agents is increasing. These drugs have a narrow, patient-specific therapeutic index, making therapeutic drug monitoring essential for safe prescribing. However, monitoring parameters vary widely, often influenced by the managing clinician’s expertise and the complexity of patients with multiple comorbidities (1). In rheumatology, this challenge is compounded by ambiguous guidelines from professional societies and regulatory bodies, as well as high monitoring costs and frequency that affect patient preferences (2). Inconsistency in recommendations about how best to monitor for adverse effects impacts which ones clinicians adhere to. Our objective was to create an evidence-based best practice paradigm for clinicians and operationalize a dynamic program for supporting care teams to avoid adverse outcomes at a tertiary care academic practice.
Methods: A large interdisciplinary steering committee was formed to assess laboratory monitoring across eight Internal Medicine specialties, with Rheumatology taking the lead due to its high patient volume. Stakeholder focus groups were conducted (among clinicians, nursing, IT, pathology, pharmacists, medical assistants and patient support advocates) to identify gaps, areas of improvement, and achieve consensus on a minimum standard of streamlined processes. A standardized laboratory monitoring policy was instituted to establish the workflows unique to each subspecialty and then operationalized at the height of COVID in 2021. Epic©, the electronic medical record (EMR), was leveraged to build a single unified platform. Smartsets were designed to facilitate efficient lab ordering, reduce manual data retrieval for staff and attain timely review of integral results.
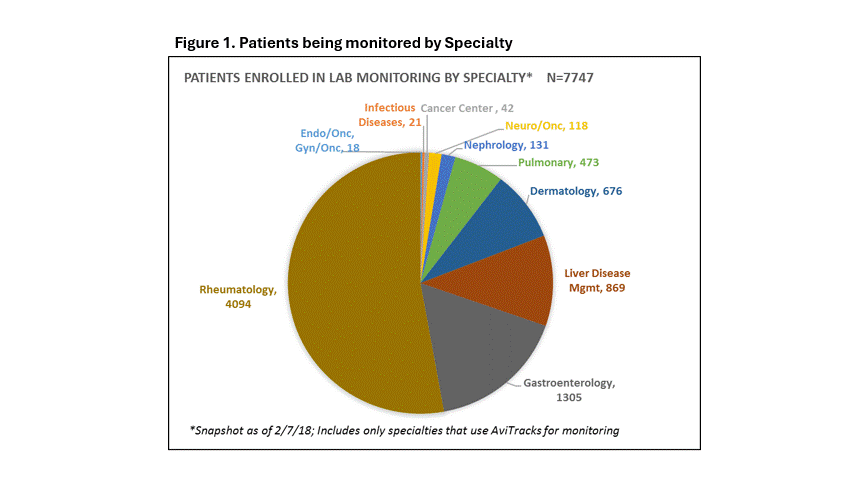
Results: Baseline data revealed 7,747 patients enrolled in various inconsistently monitored programs across IM specialties in 2020 (Figure 1). Root cause analysis identified approximately 5 % missed enrollments and several workflow gaps in the medication monitoring (Table 1). In the followup PDCA, over 16,000 patients are now being monitored for both medication safety and disease activity at our institution. We saw an incremental decline in the volume of safety events (Figure 2) from the inception of this initiative.
Conclusion: Ensuring patient safety is essential to delivering optimal care for patients with complex conditions requiring high-risk medications. Our experience at a large academic center demonstrates how the EMR can be leveraged with the support of a multi-disciplinary collaborative team to ensure successful implementation of a standardized platform to attain this longitudinal goal. A dashboard is in development for each specialty to explore the quality of key monitoring metrics in a sustainable manner.References:1. Tsakas et al. Attitudes and practices in the laboratory monitoring of conventional synthetic disease modifying anti‑rheumatic drugs by rheumatologists and rheumatology trainees BMC Rheumatology (2022) 6:59.2. Rigby WFC, Lampl K, Low JM, Furst DE. Review of routine laboratory monitoring for patients with rheumatoid arthritis receiving biologic or nonbiologic DMARDs. Int J Rheumatol. 2017;2017:9614241.
To cite this abstract in AMA style:
Khanna P, Rice M. An Epic journey – Therapeutic Drug Monitoring at an Academic Center [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/an-epic-journey-therapeutic-drug-monitoring-at-an-academic-center/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-epic-journey-therapeutic-drug-monitoring-at-an-academic-center/

.gif)
.gif)