Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patient-Reported Outcome Measures (PROMs) are integral to shared decision-making and quality improvement in rheumatology. They are recommended for monitoring treatment response, assessing quality of life, and supporting clinical care. Scientific societies as EULAR have promoted standardized of PROM collection through data set recommendations and an Outcome Measures Library with instruments and validation data https://oml.eular.org/. This study aimed to: (1) develop and implement an electronic PROM (ePROM) system within BIOBADASER, a multicenter registry of advanced therapies; (2) analyze initial results post-implementation; and (3) evaluate usability and patient satisfaction.
Methods: A core set of validated and feasible PROMs was selected based on the International Consortium for Health Outcomes Measurement (ICHOM) recommendations and expert consensus. These were incorporated into a web-based application designed for patient self-completion via internet-enabled devices (smartphone, tablet, computer). Patients initiating advanced therapies were invited to participate. A pilot phase involving six centres was conducted from December 2023 to June 2024, followed by full-scale implementation across all centres. Descriptive statistics summarized demographic, clinical, and PROM data.
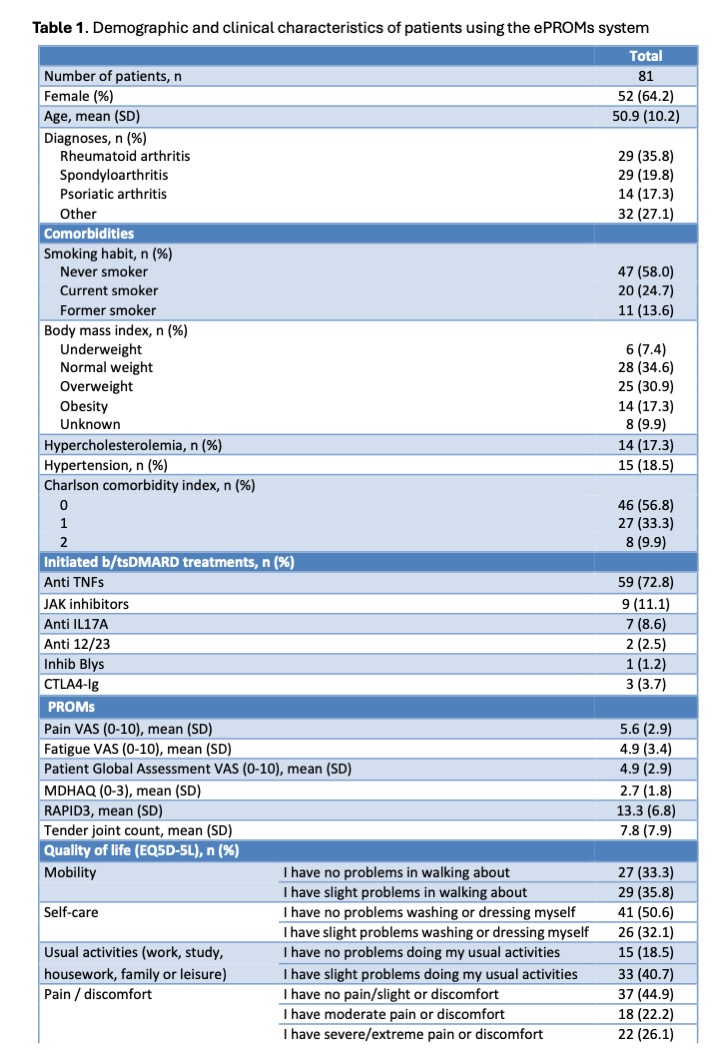
Results: From December 2023 to November 2024, 136 patients were invited to participate; 95 (70%) accessed the system, and 81 (60%) completed the questionnaires. Participants were predominantly female (64.2%) with a mean age of 50.9 years. The most common diagnoses were rheumatoid arthritis (35.8%), spondyloarthritis (19.8%), and psoriatic arthritis (17.3%). TNF inhibitors were the most frequently initiated therapy (72.8%). RAPID3 scores indicated high disease activity (mean 13.3 ± 6.8). Most patients reported minimal problems with mobility (69.1%), self-care (82.7%), usual activities (59.2%), and anxiety/depression (67.9%). The mean global health score was 55.6. Regarding usability, 81% experienced no or slight difficulty using the system, 79% found the instructions clear or very clear, and 80.2% were quite or very satisfied. A majority (66.7%) preferred online reporting over paper-based methods, and 87.6% felt comfortable using digital devices.
Conclusion: The ePROM system was feasible, well accepted, and effective for remote disease monitoring. Patients expressed high satisfaction and a preference for digital tools, supporting the integration of ePROMs into rheumatology registries to enhance personalized, patient-centred care.
To cite this abstract in AMA style:
Castrejón Fernández I, Otero L, Mera-Valera A, Garcia A, Gomez-Sabater S, Martín-Domenech R, Alvaro-Gracia J, Sarmiento-Monroy J, Ventosa B, Sánchez-Alonso F. Patient Experience and Implementation of an Electronic Patient-Reported Outcome Measures (ePROM) System for Remote Monitoring in BIOBADASER [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/patient-experience-and-implementation-of-an-electronic-patient-reported-outcome-measures-eprom-system-for-remote-monitoring-in-biobadaser/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-experience-and-implementation-of-an-electronic-patient-reported-outcome-measures-eprom-system-for-remote-monitoring-in-biobadaser/

.jpg)