Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Background: Antiphospholipid syndrome (APS) is a systemic autoimmune disorder characterized by the presence of pathogenic antiphospholipid antibodies (aPL) in the setting of venous or arterial thrombosis, pregnancy morbidity, and other morbid manifestations. B cells are central to APS pathogenesis through their role in aPL production. However, despite their pathogenic importance, the phenotypic and functional characteristics of circulating B cells in APS remain incompletely understood. In this study, we applied high-dimensional spectral flow cytometry to comprehensively characterize the activation profiles of circulating B cells in patients with APS.
Methods:
Methods: We developed a 26-color spectral flow cytometry panel to deeply characterize circulating B cell subsets and their activation states in patients with APS. The panel was designed based on established literature and included markers for B cell development, activation, memory, homing, and regulatory phenotypes. Peripheral blood mononuclear cells (PBMCs) were isolated from APS patients (n=37) fulfilling the 2023 ACR/EULAR APS classification criteria and age- and sex-matched healthy donors (n=16). Samples were stained and analyzed using a 5-laser Cytek Aurora spectral flow cytometer. Manual gating was performed using FlowJo software to define major B cell subsets, including naïve, transitional, memory, double-negative, and plasmablast populations. Activation markers such as CD86, CD95, CD69, and HLA-DR were assessed across subsets. Statistical analyses were conducted using SPSS to compare APS patients with healthy controls, with significance defined as p < 0.05.
Results:
Results: Among the patients, 25 (67.6%) were diagnosed with primary APS, while 12 (32.4%) had APS associated with systemic lupus erythematosus (SLE). Gating strategies were shown in Fig. 1. Compared with healthy controls, patients with APS exhibited an increased proportion of B1a cells (CD5+CD43+) (0.30% of CD19+CD20+B cells [0.15, 0.44] vs. 0.14 [0.08, 0.22], p=0.010) and unswitched memory B cells (CD27+IgD+IgM+) (30.52% of CD19+CD20+B cells [21.68, 36.79] vs. 12.19 [9.59, 17.58], p< 0.001). In contrast, the proportion of naïve B cells (CD27-IgD+IgM+) was significantly reduced in APS patients (46.21% of B cells [42.34, 58.44] vs. 63.01 [57.51, 72.64], p< 0.001) (Fig. 2A). Moreover, the expression of the key checkpoint regulator PD-1 was reduced on transitional B1 cells (CD10hiIgDhiCD24hiCD38hi) and unswitched memory B cells in APS patients (Fig. 2B-C).
Conclusion: High-dimensional profiling of circulating B cells in APS patients reveals distinct alterations in subset distribution and activation states, including increased B1a and unswitched memory B cells and reduced naïve B cells, alongside diminished PD-1 expression on key populations. These results provide novel insights into B cell dysregulation in APS and raise the possibility that augmenting PD-1 signaling or modulating specific B cell subsets could represent future therapeutic strategies for this complex autoimmune disorder.
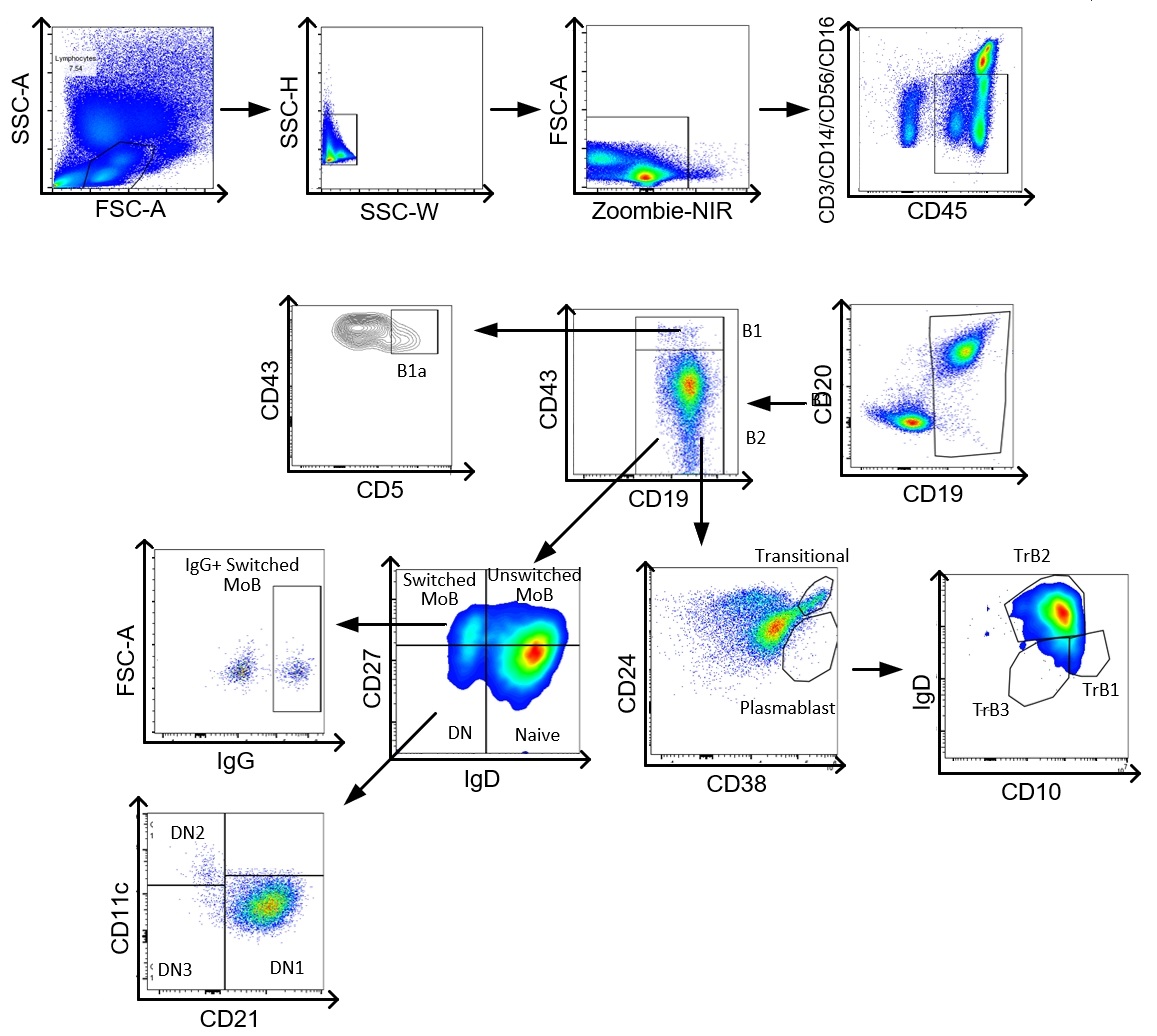 Figure 1. Gating strategies of peripheral B cells from PBMCs.
Figure 1. Gating strategies of peripheral B cells from PBMCs.
.jpg) Figure 2. Altered proportions of B cell subsets and reduced PD-1 expression on unswitched memory and transitional B1 cells in APS patients.
Figure 2. Altered proportions of B cell subsets and reduced PD-1 expression on unswitched memory and transitional B1 cells in APS patients.
(A) Heatmap showing the relative percentages (Z-score normalized) of B cell subsets in peripheral blood from patients with antiphospholipid syndrome (APS, n = 37) and healthy controls (HC, n = 16). Subsets include transitional B cells (TrB, TrB1–TrB3), memory B cells (MoB, switched and unswitched), antibody-secreting cells, naïve B cells, double-negative B cells (DN, DN1-DN3), and B1 cells (B1, B1a).
(B) Median fluorescence intensity (MFI) of PD-1 on unswitched memory B cells is significantly lower for APS patients versus HCs.
(C) PD-1 MFI on transitional B1 cells is also reduced in APS patients.
.jpg) Table 1. Baseline Demographic Characteristics of APS Patients and Healthy Controls
Table 1. Baseline Demographic Characteristics of APS Patients and Healthy Controls
To cite this abstract in AMA style:
Gan Y, Yalavarthi S, Shen Y, Sarosh C, Liu X, Tambralli A, Madison J, Zuo Y, Knight J. High-dimensional Spectral Flow Cytometry Reveals a Unique Distribution of Circulating B Cells in Patients with Antiphospholipid Syndrome [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/high-dimensional-spectral-flow-cytometry-reveals-a-unique-distribution-of-circulating-b-cells-in-patients-with-antiphospholipid-syndrome/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-dimensional-spectral-flow-cytometry-reveals-a-unique-distribution-of-circulating-b-cells-in-patients-with-antiphospholipid-syndrome/
