Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Antiphospholipid syndrome (APS) is a thrombo-inflammatory disorder that causes significant morbidity and mortality, even in children. The 2023 ACR/EULAR classification criteria, which use weighted clinical and laboratory scores, improve specificity (99% vs. 86%) but reduce sensitivity (84% vs. 99%) compared to the 2006 criteria in adults. However, no pediatric-specific criteria exist, and the 2023 criteria have not been validated in children. This multisite North American study aims to assess the performance of both the 2023 and 2006 criteria in pediatric APS.
Methods: Patients with pediatric APS and childhood-onset SLE were initially identified by rheumatologists at 5 academic institutions through electronic medical record searches using diagnosis codes. APS diagnosis was determined by documentation of a confirmed diagnosis at age ≤18 by a pediatric hematologist or rheumatologist. SLE controls had a confirmed diagnosis by age 18 and ≥2 antiphospholipid antibody (aPL) test results available. Analyses were performed using Python (v3.10).
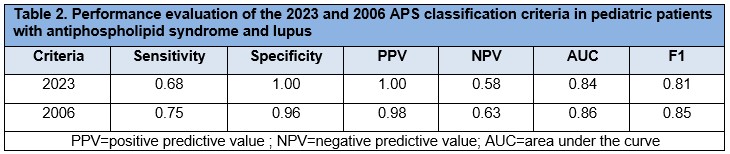
Results: We evaluated the performance of the adult 2023 and 2006 APS classification criteria in a well-characterized, multicenter cohort of pediatric patients diagnosed with APS (n=57) and lupus (n=25) (Table 1). When applied to pediatric patients, the 2023 criteria demonstrated improved but similar specificity compared with the 2006 version (100% vs. 96%) and reduced sensitivity (68% vs. 75%). The 2023 criteria also showed a slightly higher positive predictive value (PPV) for diagnosis, reflecting a modest increase in precision (100% vs. 98%), but lower negative predictive value (NPV, 58% vs. 63%). The F1 score, reflecting overall diagnostic performance, was also lower with the 2023 criteria (0.81 vs. 0.85). ROC analysis favored the 2006 criteria (AUC = 0.86 vs. 0.84) (Table 2). Both criteria demonstrated strong agreement (Cohen’s kappa = 0.88). Among the 18 APS patients who failed to meet the 2023 criteria (including all 14 who also failed the 2006 criteria), 4 did not reach the clinical criteria threshold, and 14 lacked sufficient laboratory points—8 of whom had only a single positive lupus anticoagulant test. Of the 4 patients meeting the 2006 but not the 2023 criteria, 2 failed to meet clinical and 2 failed to meet lab thresholds. To better understand the profile of missed cases, we compared domain scores between false negatives and the overall cohort. False negatives under the 2006 criteria had significantly lower laboratory scores (p = 0.033), suggesting under-recognition of patients with weaker serologic profiles. No significant differences were observed in clinical scores.
Conclusion: Both the 2006 and 2023 APS criteria perform reasonably well in children but with lower sensitivity than in adults. The 2006 criteria are more sensitive—especially for cases with milder, lower-scoring aPL profiles—while the 2023 criteria offer greater specificity. Their high concordance supports the reliability of both systems, but also underscores the need to refine adult-based criteria to better capture true pediatric APS. Our ongoing multisite study (target n=300 across 7 sites), using expert-confirmed diagnoses, will help guide optimal classification in this population.
To cite this abstract in AMA style:
Madison J, Sloan E, Saez C, Kwan O, Lewis K, Marilao J, Baay B, Elrefai R, Dale M, McCurdy D, Bhatt J, Goteti S, Ogbu E, Knight J, Zuo Y. Performance of the 2023 and 2006 APS Classification Criteria in Pediatric Patients Diagnosed with APS: A Multisite Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/performance-of-the-2023-and-2006-aps-classification-criteria-in-pediatric-patients-diagnosed-with-aps-a-multisite-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-the-2023-and-2006-aps-classification-criteria-in-pediatric-patients-diagnosed-with-aps-a-multisite-cohort-study/

.jpg)