Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Although antiphospholipid antibodies (aPL) are well-established risk factors for thrombosis, heritable thrombophilias (HT) are also associated with venous thromboembolism (VTE). The latter includes deficiencies of the naturally occurring anticoagulants (antithrombin, protein C, and protein S), and factor V Leiden (FVL) and prothrombin (PT) G20210A gene mutations. Methylene tetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms are not considered part of the HT screen in clinical practice. The primary objective of this ongoing study was to determine the prevalence of HT in persistently aPL-positive patients. Secondly, we analyzed the association between thrombosis and HT in aPL-positive patients, individually and in combination. TRANSLATE with xEnglishArabicHebrewPolishBulgarianHindiPortugueseCatalanHmong DawRomanianChinese SimplifiedHungarianRussianChinese TraditionalIndonesianSlovakCzechItalianSlovenianDanishJapaneseSpanishDutchKlingonSwedishEnglishKoreanThaiEstonianLatvianTurkishFinnishLithuanianUkrainianFrenchMalayUrduGermanMalteseVietnameseGreekNorwegianWelshHaitian CreolePersian TRANSLATE with COPY THE URL BELOW BackEMBED THE SNIPPET BELOW IN YOUR SITE Enable collaborative features and customize widget: Bing Webmaster PortalBack This page is in English Translate to Turkish AfrikaansAlbanianAmharicArabicArmenianAzerbaijaniBengaliBulgarianCatalanCroatianCzechDanishDutchEnglishEstonianFinnishFrenchGermanGreekGujaratiHaitian CreoleHebrewHindiHungarianIcelandicIndonesianItalianJapaneseKannadaKazakhKhmerKoreanKurdish (Kurmanji)LaoLatvianLithuanianMalagasyMalayMalayalamMalteseMaoriMarathiMyanmar (Burmese)NepaliNorwegianPashtoPersianPolishPortuguesePunjabiRomanianRussianSamoanSimplified ChineseSlovakSlovenianSpanishSwedishTamilTeluguThaiTraditional ChineseTurkishUkrainianUrduVietnameseWelsh Always translate English to Turkish Never translate English Never translate www.abstractscorecard.com
Methods: A web-based data capture system is used to store patient demographics and aPL-related medical history in APS ACTION registry. Inclusion criteria are positive aPL, based on the Revised Sapporo Antiphospholipid Syndrome (APS) classification criteria, tested at least twice within one year prior to enrollment. Patients are followed every 12±3 months with clinical data and blood collection. For this cross-sectional analysis, baseline data were used including demographic, clinical, and HT laboratory (either center-reported [CR] or APS ACTION United Kingdom Core Laboratory [CL] data (following testing in the CL)). When evaluating the results of the HT included (see Table 1), CL were used over CR if both were available; Chi square or Fisher’s exact tests were used for comparisons. TRANSLATE with xEnglishArabicHebrewPolishBulgarianHindiPortugueseCatalanHmong DawRomanianChinese SimplifiedHungarianRussianChinese TraditionalIndonesianSlovakCzechItalianSlovenianDanishJapaneseSpanishDutchKlingonSwedishEnglishKoreanThaiEstonianLatvianTurkishFinnishLithuanianUkrainianFrenchMalayUrduGermanMalteseVietnameseGreekNorwegianWelshHaitian CreolePersian TRANSLATE with COPY THE URL BELOW BackEMBED THE SNIPPET BELOW IN YOUR SITE Enable collaborative features and customize widget: Bing Webmaster PortalBack This page is in English Translate to Turkish AfrikaansAlbanianAmharicArabicArmenianAzerbaijaniBengaliBulgarianCatalanCroatianCzechDanishDutchEnglishEstonianFinnishFrenchGermanGreekGujaratiHaitian CreoleHebrewHindiHungarianIcelandicIndonesianItalianJapaneseKannadaKazakhKhmerKoreanKurdish (Kurmanji)LaoLatvianLithuanianMalagasyMalayMalayalamMalteseMaoriMarathiMyanmar (Burmese)NepaliNorwegianPashtoPersianPolishPortuguesePunjabiRomanianRussianSamoanSimplified ChineseSlovakSlovenianSpanishSwedishTamilTeluguThaiTraditional ChineseTurkishUkrainianUrduVietnameseWelsh Always translate English to Turkish Never translate English Never translate www.abstractscorecard.com
Results: Of 1,252 patients recruited as of January 2025, 741 (59%) had at least one CR and/or CL HT result (female: 515 [70%]; mean age at registry entry: 55±13.5; thrombotic APS: 456 [62%]; triple aPL-positive: 273 [37%], lupus anticoagulant with/without aCL or aβ2GPI: 308 [42%]). The prevalence of each HT and the association with vascular events are shown in Tables 1 and 2, respectively. Homozygosity for the MTHFR C677T and A1286 polymorphisms were reported in 20 (6%) and 4 (1%), respectively, with high homocysteine in 2/20 with MTHFR C677T and none with MTHFR A1298C. In the subgroup analysis of 316 (43%) patients, we compared those with at least one abnormal HT result (n: 86; excluding heterozygous HT) and those with normal results for all HT assessed (n=230); the former group had significantly more VTE, but not arterial, events (p=0.015) (Table 3). TRANSLATE with xEnglishArabicHebrewPolishBulgarianHindiPortugueseCatalanHmong DawRomanianChinese SimplifiedHungarianRussianChinese TraditionalIndonesianSlovakCzechItalianSlovenianDanishJapaneseSpanishDutchKlingonSwedishEnglishKoreanThaiEstonianLatvianTurkishFinnishLithuanianUkrainianFrenchMalayUrduGermanMalteseVietnameseGreekNorwegianWelshHaitian CreolePersian TRANSLATE with COPY THE URL BELOW BackEMBED THE SNIPPET BELOW IN YOUR SITE Enable collaborative features and customize widget: Bing Webmaster PortalBack This page is in English Translate to Turkish AfrikaansAlbanianAmharicArabicArmenianAzerbaijaniBengaliBulgarianCatalanCroatianCzechDanishDutchEnglishEstonianFinnishFrenchGermanGreekGujaratiHaitian CreoleHebrewHindiHungarianIcelandicIndonesianItalianJapaneseKannadaKazakhKhmerKoreanKurdish (Kurmanji)LaoLatvianLithuanianMalagasyMalayMalayalamMalteseMaoriMarathiMyanmar (Burmese)NepaliNorwegianPashtoPersianPolishPortuguesePunjabiRomanianRussianSamoanSimplified ChineseSlovakSlovenianSpanishSwedishTamilTeluguThaiTraditional ChineseTurkishUkrainianUrduVietnameseWelsh Always translate English to Turkish Never translate English Never translate www.abstractscorecard.com
Conclusion: Based on our large-scale international cohort, up to six percent of persistently aPL-positive patients may have one or more heritable thrombophilia (antithrombin, protein C, or protein S deficiencies, FVL or PT G20210A), which may increase the risk of VTE. Our preliminary results have limitations, including reporting/selection bias, and the lack of information on testing methodology/confirmation and factors that may affect results of naturally occurring anticoagulants, e.g. anticoagulation and pregnancy/puerperium. However, these results and the relatively high frequency of HT in the general population highlight the potential additive risk of HT in aPL-positive patients that may be contributory in the stratification and management of such patients. TRANSLATE with xEnglishArabicHebrewPolishBulgarianHindiPortugueseCatalanHmong DawRomanianChinese SimplifiedHungarianRussianChinese TraditionalIndonesianSlovakCzechItalianSlovenianDanishJapaneseSpanishDutchKlingonSwedishEnglishKoreanThaiEstonianLatvianTurkishFinnishLithuanianUkrainianFrenchMalayUrduGermanMalteseVietnameseGreekNorwegianWelshHaitian CreolePersian TRANSLATE with COPY THE URL BELOW BackEMBED THE SNIPPET BELOW IN YOUR SITE Enable collaborative features and customize widget: Bing Webmaster PortalBack This page is in English Translate to Turkish AfrikaansAlbanianAmharicArabicArmenianAzerbaijaniBengaliBulgarianCatalanCroatianCzechDanishDutchEnglishEstonianFinnishFrenchGermanGreekGujaratiHaitian CreoleHebrewHindiHungarianIcelandicIndonesianItalianJapaneseKannadaKazakhKhmerKoreanKurdish (Kurmanji)LaoLatvianLithuanianMalagasyMalayMalayalamMalteseMaoriMarathiMyanmar (Burmese)NepaliNorwegianPashtoPersianPolishPortuguesePunjabiRomanianRussianSamoanSimplified ChineseSlovakSlovenianSpanishSwedishTamilTeluguThaiTraditional ChineseTurkishUkrainianUrduVietnameseWelsh Always translate English to Turkish Never translate English Never translate www.abstractscorecard.com
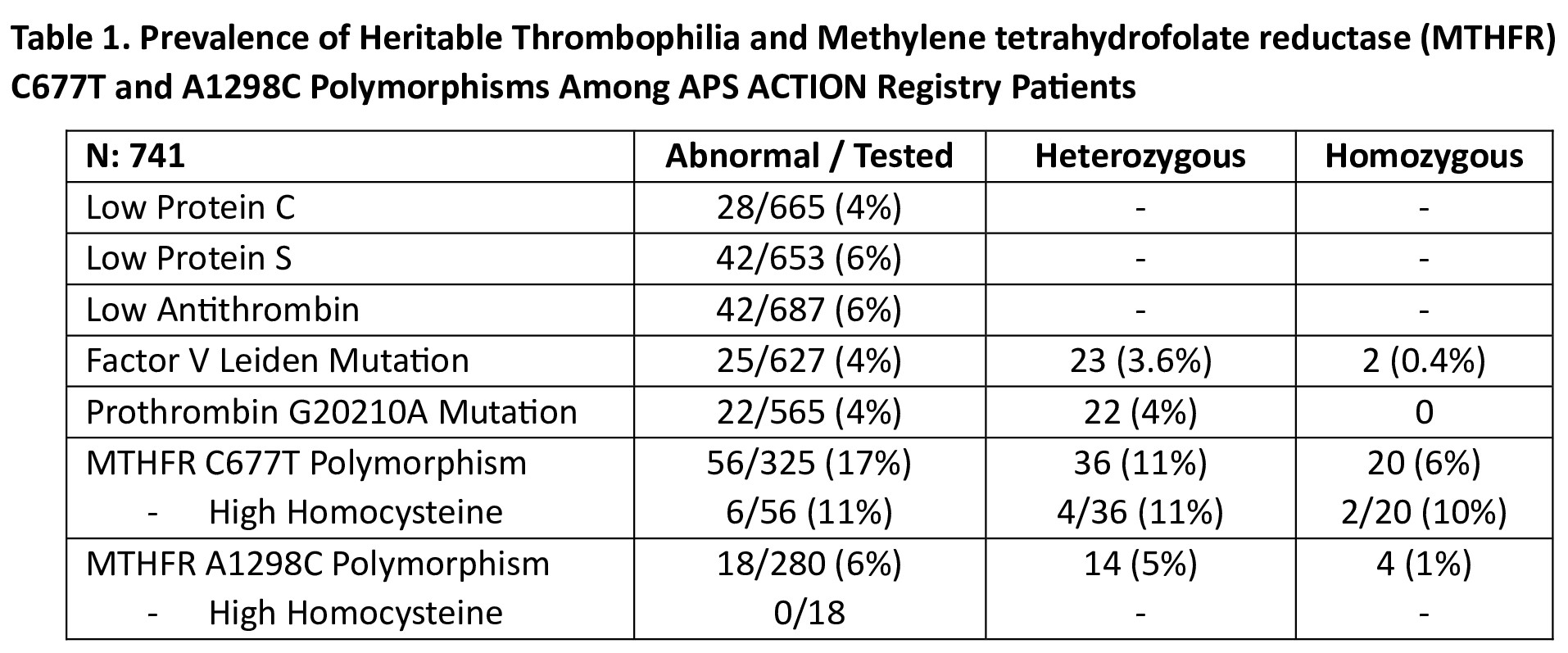 Table 1. Prevalence of Heritable Thrombophilia and Methylene tetrahydrofolate reductase (MTHFR) C677T and A1298C Polymorphisms Among APS ACTION Registry Patients
Table 1. Prevalence of Heritable Thrombophilia and Methylene tetrahydrofolate reductase (MTHFR) C677T and A1298C Polymorphisms Among APS ACTION Registry Patients
.jpg) Table 2. The Frequency of Arterial/Venous Thrombosis is Patients with Individual Heritable Thrombophilia and Methylene tetrahydrofolate reductase (MTHFR) C677T and A1298C Polymorphisms Among APS ACTION Registry Patients (those with heterozygous MTHFR polymorphisms were not included)
Table 2. The Frequency of Arterial/Venous Thrombosis is Patients with Individual Heritable Thrombophilia and Methylene tetrahydrofolate reductase (MTHFR) C677T and A1298C Polymorphisms Among APS ACTION Registry Patients (those with heterozygous MTHFR polymorphisms were not included)
.jpg) Table 3. Comparison of Arterial and Venous Events Between Patients With and Without Heritable Thrombophilia (HT) Among APS ACTION Registry Patients (those with MTHFR polymorphisms were not included)
Table 3. Comparison of Arterial and Venous Events Between Patients With and Without Heritable Thrombophilia (HT) Among APS ACTION Registry Patients (those with MTHFR polymorphisms were not included)
To cite this abstract in AMA style:
Sahin E, Efthymiou M, Andrade D, Barber M, Tektonidou M, Pengo V, Radin M, Pardos-Gea J, AGUIRRE ZAMORANO M, Kello N, Paredes-Ruiz D, Belmont H, Fortin P, WAHL D, Branch W, Gerosa M, Ramires de Jesus G, Zhang Z, Atsumi T, Pazzola G, Andreoli L, Duarte-Garcia A, Rodriguez-Almaraz E, Petri M, Cervera R, Artim Esen B, Pons-Estel G, Shi H, Knight J, Willis R, Bertolaccini M, Cohen H, Erkan D. The Prevalence and Clinical Significance of Heritable Thrombophilia in Antiphospholipid Antibody Positive Patients: Descriptive Results from the Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-prevalence-and-clinical-significance-of-heritable-thrombophilia-in-antiphospholipid-antibody-positive-patients-descriptive-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-prevalence-and-clinical-significance-of-heritable-thrombophilia-in-antiphospholipid-antibody-positive-patients-descriptive-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials/
