Session Information
Date: Sunday, October 26, 2025
Title: (0098–0114) Spondyloarthritis Including Psoriatic Arthritis – Basic Science Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Iron metabolism dysregulation has been increasingly recognized as a key factor in the pathogenesis of inflammatory arthritis, with evidence linking altered iron homeostasis to disease progression. However, the mechanisms through which iron dysregulation contributes to inflammation and tissue remodeling in inflammatory arthritis, particularly in ankylosing spondylitis, remain poorly understood. This study aims to investigate the role of iron metabolism dysregulation, particularly the inflammatory mediator SLC39A14, in driving extracellular matrix (ECM) remodeling and ectopic ossification in inflammatory arthritis, using ankylosing spondylitis as a model.
Methods: Primary fibroblasts were isolated from spinal ligament tissues of AS patients, and THP-1 cells were differentiated into macrophages for in vitro studies. The CAIA and SKG mouse models were used, with ferric ammonium citrate (FAC) injected locally in the SKG model to induce iron overload. SLC39A14 conditional knockout (CKO) mice were generated by crossing SLC39A14-flox mice with SCX-cre mice. Ferritin levels and plasma iron concentrations were measured using a colorimetric assay. Immunohistochemistry and immunofluorescence were performed to assess co-localization of Ferritin in fibroblasts and macrophages. CRISPR-Cas9 was used to knock out SLC39A14 in the TTD6 fibroblast cell line, and gene editing efficiency was confirmed by PCR and western blotting. FerroOrange, combined with flow cytometry, assessed iron uptake in treated cells. In vivo, the effects of iron overload were evaluated in the CAIA and SKG models, with western blotting to analyze SLC39A14, Ferritin, and ECM-related proteins.
Results: Our research demonstrated that inflammation-induced iron overload in spinal ligaments led to ECM remodeling and ectopic ossification. Increased Ferritin levels and decreased plasma iron were observed in AS patients, with serum iron negatively correlated with ASDAS-CRP scores, linking iron metabolism to disease severity. TNFα stimulation upregulated SLC39A14 in fibroblasts, promoting excessive ECM deposition. SLC39A14 knockout in fibroblasts inhibited ECM-related gene expression, such as TNC, FN1, and collagen types I and III, highlighting its role in ECM remodeling. Immunohistochemistry showed co-localization of SLC39A14 and Ferritin in activated fibroblasts and macrophages. Single-cell RNA sequencing identified fibroblast subpopulations sensitive to iron accumulation, with elevated ECM gene expression. In vivo, FAC injections in the SKG mouse model induced ectopic ossification in the Achilles tendon, further supporting the role of iron overload in ECM remodeling.
Conclusion: In an inflammatory state, upregulation of SLC39A14 in fibroblasts leads to iron overload, driving ECM remodeling and ectopic ossification in inflammatory arthritis, such as ankylosing spondylitis. Targeting SLC39A14 and modulating iron metabolism may offer potential therapeutic strategies to prevent these pathological changes.
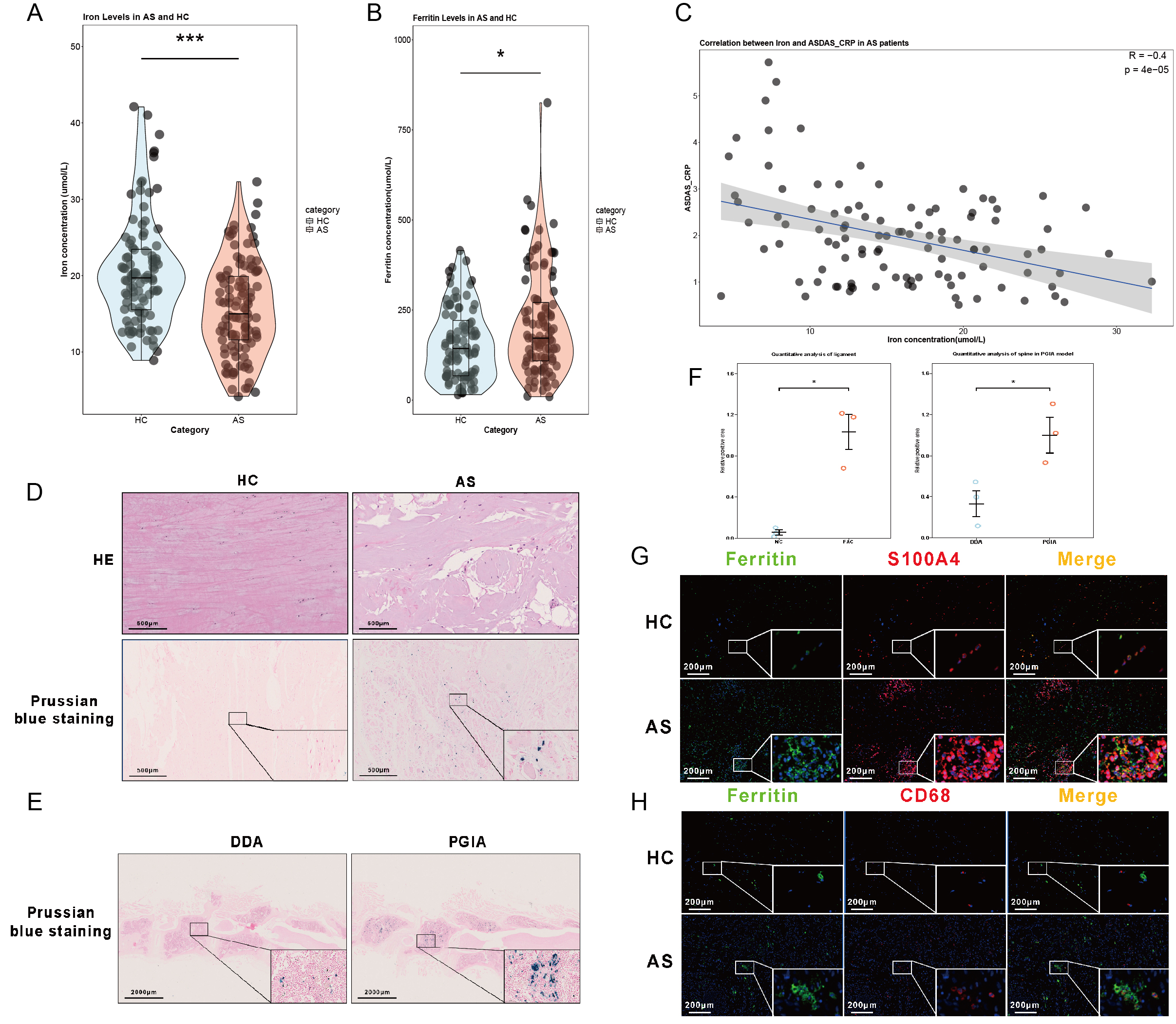 FIGURE 1. Iron Accumulation in Spinal Ligaments of Patients with Ankylosing Spondylitis (AS)
FIGURE 1. Iron Accumulation in Spinal Ligaments of Patients with Ankylosing Spondylitis (AS)
(A) Violin plot showing significantly lower serum iron levels in AS patients compared to healthy controls (HC), with statistical significance (p < 0.05).
(B) Violin plot displaying higher Ferritin concentrations in AS patients relative to HC (p < 0.05).
(C) Correlation between serum iron levels and ASDAS-CRP scores in AS patients, showing a negative correlation (r = -0.4, p = 4e-5).
(D) Hematoxylin and eosin (HE) staining and Prussian blue staining of spinal ligament tissues, highlighting iron deposits (blue staining) in AS compared to HC. Scale bars: 50 µm (HE) and 500 µm (Prussian blue).
(E) Prussian blue staining of spinal ligaments in the DDA (control group, Dimethyl-dioctadecyl-ammonium) and PGIA (Proteoglycan- induced arthritis) models, showing iron accumulation. Scale bars: 200 µm.
(F) Quantification of iron levels and expression of ECM-related genes, demonstrating significant differences (p < 0.05).
(G) Immunofluorescence staining for Ferritin (green) and S100A4 (red) in spinal ligaments, showing co-localization of Ferritin with activated fibroblasts (S100A4) in AS compared to HC. Scale bars: 200 µm.
(H) Immunofluorescence staining for Ferritin (green) and CD68 (red) in spinal ligaments, showing co-localization of Ferritin with macrophages (CD68) in AS compared to HC. Scale bars: 200 µm.
.jpg) FIGURE 2. Single-Cell Sequencing Reveals Iron Accumulation and Cellular Heterogeneity in Spinal Ligaments of Ankylosing Spondylitis (AS)
FIGURE 2. Single-Cell Sequencing Reveals Iron Accumulation and Cellular Heterogeneity in Spinal Ligaments of Ankylosing Spondylitis (AS)
(A) Schematic representation of single-cell sequencing analysis on spinal ligament tissues from healthy and AS patients. Stromal and immune cell populations were identified and classified into subtypes, with key genes and cell types highlighted.
(B) UMAP plot showing the clustering of different cell populations in the spinal ligaments of AS patients, including endothelial cells, pericytes, fibroblasts, myeloid cells, and T/B cells. Each cluster is numbered and color-coded.
(C) Heatmap of differentially expressed genes in various cell populations, illustrating key biological processes related to immune response, ECM remodeling, and metabolic pathways. Gene expression patterns in different cell types are shown, with color-coded gene categories.
(D) Boxplot showing the iron accumulation signature (IAS) across different cell populations, indicating the extent of iron accumulation in various subtypes of cells within the spinal ligaments.
.jpg) FIGURE 3. Iron Accumulation and ECM Remodeling in Ankylosing Spondylitis (AS) Revealed by Single-Cell Sequencing and In Vivo Imaging
FIGURE 3. Iron Accumulation and ECM Remodeling in Ankylosing Spondylitis (AS) Revealed by Single-Cell Sequencing and In Vivo Imaging
(A) UMAP plot showing the clustering of different cell populations in the spinal ligaments of AS patients, including subpopulations associated with iron metabolism.
(B) Expression of iron-related genes across different cell types in AS and control samples. Genes involved in iron storage, uptake, traffic, and export are shown with varying expression patterns between AS and control groups.
(C) Bubble plot highlighting significantly enriched pathways in AS patients, with a focus on iron metabolism, ECM remodeling, and related inflammatory pathways.
(D) Correlation analysis of iron and collagen accumulation in AS patient samples, showing that increased iron accumulation is associated with higher collagen accumulation in spinal ligaments.
(E) Western blot analysis of ECM-related proteins (FN1, TNC, COL I, COL III) in fibroblasts treated with ferric ammonium citrate (FAC) and/or deferoxamine (DFO), demonstrating the effect of iron overload on ECM protein expression.
(F) Micro-CT images of SKG mice showing ectopic ossification in the Achilles tendon following FAC injection, with the arrows indicating the regions of ossification. tv, top view. lv, lateral view. Scale bars: 5 mm.
To cite this abstract in AMA style:
Zhuo D, Tang Y, Ma X, Geng C, Xie J, Wang J, Liu J. Iron Metabolism Dysregulation and Inflammation in Ankylosing Spondylitis: Role of SLC39A14 in Extracellular Matrix Remodeling [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/iron-metabolism-dysregulation-and-inflammation-in-ankylosing-spondylitis-role-of-slc39a14-in-extracellular-matrix-remodeling/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/iron-metabolism-dysregulation-and-inflammation-in-ankylosing-spondylitis-role-of-slc39a14-in-extracellular-matrix-remodeling/
