Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Human leukocyte antigen (HLA) class II genes play a central role in antigen presentation and immune modulation. The shared epitope (SE) region within HLA-DRB1 is a well-established genetic risk factor for rheumatoid arthritis (RA) and is associated with anti-citrullinated protein antibody (ACPA) positivity. RA is a heterogeneous disease, with some individuals developing ACPA positivity years before clinical symptoms, while others seroconvert only after disease onset or remain seronegative. Although prior studies have linked individual HLA alleles with disease risk, there are no composite genetic scores exist that capture HLA-driven risk for key disease features, such as RA stage progression or the emergence of ACPA positivity.
Methods: Using the Accelerating Medicines Partnership (AMP) cross-sectional dataset (n=349, Inamo et. al, JCI 2025), we developed two HLA-based genetic risk scores targeting distinct outcomes: (1) natural RA progression across clinical stages (control, at-risk, RA), and (2) ACPA positivity, defined as CCP3.0 and/or CCP3.1 >20 units. At-risk was defined as being either ACPA+ and/or having a first degree relative (FDR) with RA. Genotyping was performed using the Illumina Multi-Ethnic Genotyping Array, and classical HLA alleles were imputed using the TOPMed reference panel. We restricted analyses to participants of European ancestry (n=153, Table 1). Each score was derived using a three-step pipeline: (1) individual regression models performed for each imputed HLA allele, modeling both additive (0/1/2 copies) and heterozygous genotype effects, while adjusting for sex, age, and the first five genetic principal components. For the RA progression score, we used ordinal logistic regression with RA stage (control, ACAP positive at-risk, early seropositive RA) as the outcome, while the ACPA positivity score used a traditional logistic model with ACPA positivity (yes/no) regardless of RA status as the outcome and additionally adjusted for RA status in the model (Figure 1). (2) Markers with FDR-adjusted p-values < 0.05 from likelihood ratio tests were entered into a 5-fold cross-validated elastic net model. (3) Resulting coefficients were used to calculate a weighted genetic risk score. Each score was trained on 80% of the data and tested in the remaining 20%.
Results: Figure 2 depicts the distributions of the two HLA risk scores. The RA progression score showed strong performance with AUCs of 0.900 (early RA vs control), 0.708 (at-risk vs control), and 0.791 (early RA vs at-risk) in the testing set. The ACPA positivity score also demonstrated moderate discriminative ability, with an AUC of 0.671 (ACPA+ vs ACPA−). Each score included multiple markers beyond traditional SE alleles and incorporated both additive and heterozygous effects, allowing for more nuanced modeling of HLA-driven risk.
Conclusion: We developed and internally validated two HLA-based genetic risk scores that distinguish between stages of RA progression and predict ACPA positivity within a single cross-sectional cohort. These tools provide a foundation for improved HLA-based stratification and may help clarify the genetic mechanisms underlying distinct features of RA development.
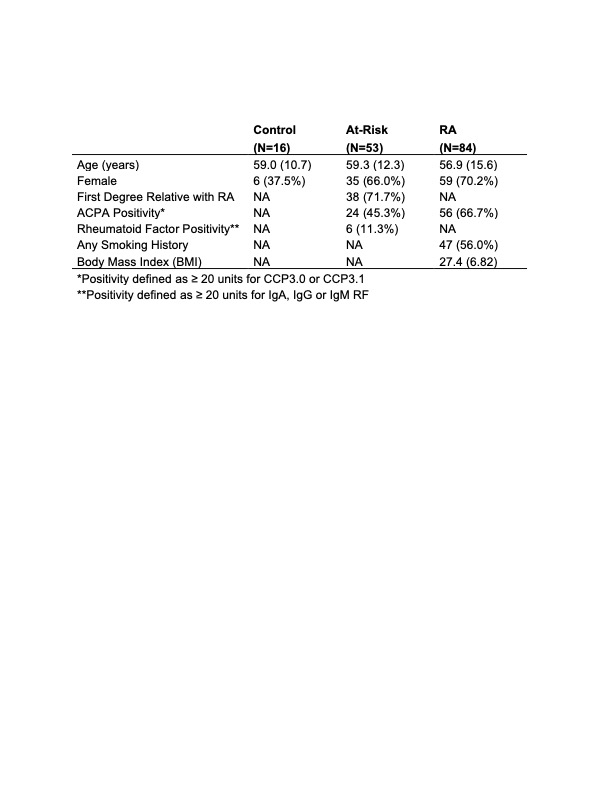 Table 1. Demographic characteristics of European ancestry participants in AMP.
Table 1. Demographic characteristics of European ancestry participants in AMP.
Demographic characteristics are shown for the 153 participants of European ancestry in the AMP study, stratified by original clinical group (control, at-risk, or early RA). Data are presented as mean (standard deviation) for continuous variables and N (%) for categorical variables.
.jpg) Figure 1. Study design and HLA risk score development pipeline.
Figure 1. Study design and HLA risk score development pipeline.
A. Participants with European ancestry from the AMP cohort were used to train and internally validate (test) a genetic risk score for RA progression and ACPA positivity based on HLA allele markers. The AMP dataset includes controls, at-risk (ACPA-positive and/or first degree relative with RA) individuals, and early RA cases (both seropositive and seronegative). ACPA negative individuals were removed from the at-risk and RA groups in the disease progression risk-score development, while all individuals were used in the ACPA positivity risk-score, but groups defined based on ACPA-positivity status and not the original group.
B. Each HLA risk score was constructed using a three-step pipeline: (1) FDR-filtered regression (either ordinal logistic or logistic for the RA progression and ACPA positivity risk scores respectively), adjusting for age, sex, and the first five genetic PCs (RA status was an additional covariate in the ACPA positivity model); to identify outcome-associated HLA allele markers; (2) 5-fold cross-validated elastic net modeling to select and weight markers; and (3) weighted summation of coefficients to compute the HLA risk score. The scores were internally validated with the 20% testing dataset.
.jpg) Figure 2. Distribution of Two HLA Risk Scores.
Figure 2. Distribution of Two HLA Risk Scores.
A. Histogram and boxplot representations of the RA progression HLA risk score, stratified by disease stage (control, ACPA-positive at-risk, and ACPA-positive early RA), which was the outcome used to train the score.
B. Histogram and boxplot representations of the ACPA positivity HLA risk score, stratified by ACPA positivity status (no vs yes), which was the binary outcome used to train the score.
To cite this abstract in AMA style:
Vanderlinden L, Inamo J, Seifert J, Holers V, Guthridge J, Deane K, Zhang F. Development and Internal Validation of Two Human Leukocyte Antigen Genetic Risk Scores for Predicting Rheumatoid Arthritis Progression and Anti-Citrullinated Protein Antibody-Positivity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-and-internal-validation-of-two-human-leukocyte-antigen-genetic-risk-scores-for-predicting-rheumatoid-arthritis-progression-and-anti-citrullinated-protein-antibody-positivity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-internal-validation-of-two-human-leukocyte-antigen-genetic-risk-scores-for-predicting-rheumatoid-arthritis-progression-and-anti-citrullinated-protein-antibody-positivity/
