Session Information
Date: Sunday, October 26, 2025
Title: (0001–0018) B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: B cell depletion is a validated therapeutic strategy in autoimmune disease, but current approaches targeting CD20 may only partially eliminate disease-driving subsets such as early lineage B cells and plasma cells. T-cell engagers (TCEs) that redirect cytotoxic T cells to B-lineage targets offer a promising path to achieve deeper and more durable depletion. XmAb657 is a CD19 x CD3 bispecific antibody specifically engineered for autoimmune indications. CD19 is broadly expressed across the B cell lineage, including on subsets not addressed by CD20-targeted therapies. XmAb657 incorporates a bivalent CD19-binding arm to enhance avidity and improve engagement of CD19+ B cells, while the TCE format facilitates potent T cell–mediated cytotoxicity, including in tissue compartments where conventional therapies may have limited activity. XmAb657 was designed using a clinically validated 2+1 bispecific format and an Fc domain engineered for extended half-life.
Methods: XmAb657 was tested in a human PBMC-based redirected T cell cytotoxicity (RTCC) assay to assess B cell killing, cytokine production (IFNγ), and T cell activation (CD69, CD25). In vivo pharmacokinetics, pharmacodynamics, and tissue depletion were evaluated in cynomolgus monkeys following a single intravenous (IV) or subcutaneous (SC) dose. Peripheral blood, bone marrow, and lymph node B cells and plasma cells were measured for up to 42 days. Extent of lymph node B cell depletion was also assessed by IHC using a non-competing anti-CD19 antibody.
Results: In vitro, XmAb657 induced potent B cell cytotoxicity and T cell activation at low nanomolar concentrations. In cynomolgus monkeys, a single IV or SC dose of XmAb657 led to rapid and sustained depletion of peripheral B cells, and B cell counts were suppressed to levels below reliable quantitation (< 5 B cells/µL blood) for at least 42 days. Tissue analysis confirmed marked reductions in CD19+ B cells as well as CD19+ plasma cells in both lymph nodes and bone marrow. Serum half-life was approximately 15 days. XmAb657 was well tolerated in cynomolgus monkeys, with no clinical signs consistent with cytokine release.
Conclusion: XmAb657, a CD19 x CD3 bispecific antibody engineered for treatment of autoimmune disease, demonstrated potent in vitro activity and deep, durable depletion of circulating and tissue-resident B cells in cynomolgus monkeys after a single dose. Targeting CD19 may complement other B-lineage–directed approaches by extending depletion to a broader range of B cell subsets. These findings support the ongoing advancement of XmAb657 into first-in-human clinical studies.
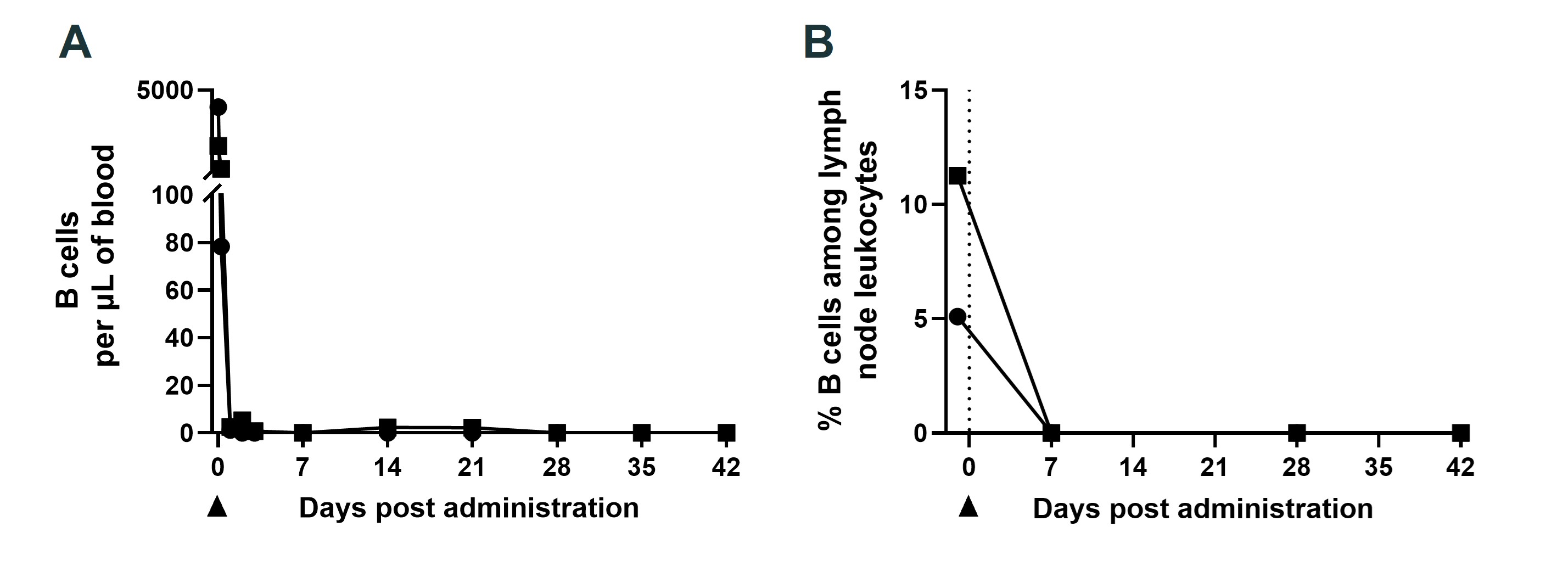 Figure 1. B cell depletion in cynomolgus monkeys (n = 2) following a single intravenous dose of XmAb657. (A) Peripheral blood B cell counts, expressed as absolute cells/μL. (B) Lymph node B cells, expressed as percentage of total leukocytes. B cells were quantified by flow cytometry.
Figure 1. B cell depletion in cynomolgus monkeys (n = 2) following a single intravenous dose of XmAb657. (A) Peripheral blood B cell counts, expressed as absolute cells/μL. (B) Lymph node B cells, expressed as percentage of total leukocytes. B cells were quantified by flow cytometry.
To cite this abstract in AMA style:
Bernett M, Moore G, Bykova K, Davra V, Chu S, Sheard M, Love R, Barlow N, Salama E, Kanodia J, Lertkiatmongkol P, Chaudhuri D, Avery K, Nguyen H, Rashid R, Liu K, Qi J, Eivazi A, Truong T, Karki S, Ernst J, Bahjat R, Desjarlais J. XmAb657, a CD19 x CD3 T-Cell Engaging Bispecific Antibody for Autoimmune Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/xmab657-a-cd19-x-cd3-t-cell-engaging-bispecific-antibody-for-autoimmune-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/xmab657-a-cd19-x-cd3-t-cell-engaging-bispecific-antibody-for-autoimmune-disease/
