Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Tofacitinib (TOF) has been shown to be efficacious in the treatment of polyarticular course JIA, including systemic JIA (sJIA) without active systemic features. Here we report efficacy/safety/tolerability of TOF in participants (pts) with sJIA with active disease.
Methods: This was a Phase 3, global, 2-phase, randomized withdrawal study (NCT03000439), enrolling pts aged 2–< 18 years with active sJIA. In the open-label (OL) phase, pts were treated with TOF (5 mg twice daily or equivalent weight-based dose): in Part 1 (up to 16 wks), pts had to achieve and maintain an adapted (a) JIA-ACR30 response for ≥4 wks to proceed to OL Part 2 (up to 24 wks), in which pts treated with CS ˃0.2 mg/kg/day attempted to taper while maintaining clinical response. Responders (aJIA-ACR30, tapered CS) were eligible to enter the double-blind (DB) phase and were randomized to either continue TOF or switch to placebo (PBO). The primary endpoint was time to sJIA disease flare in the DB phase. Secondary efficacy endpoints and safety were assessed during the OL and DB phases. A prespecified interim analysis was performed after 28 pts reported an sJIA flare in the DB phase.
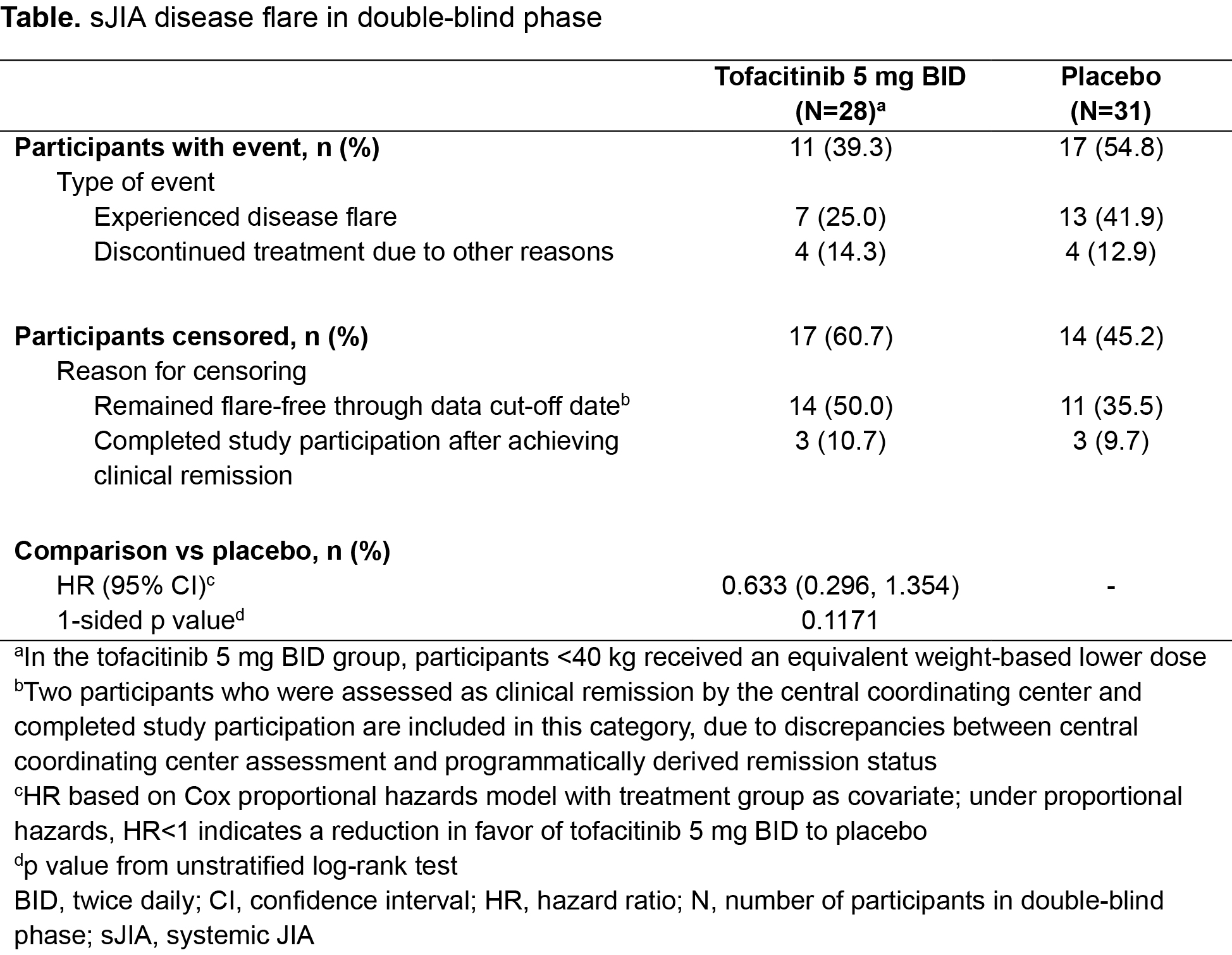
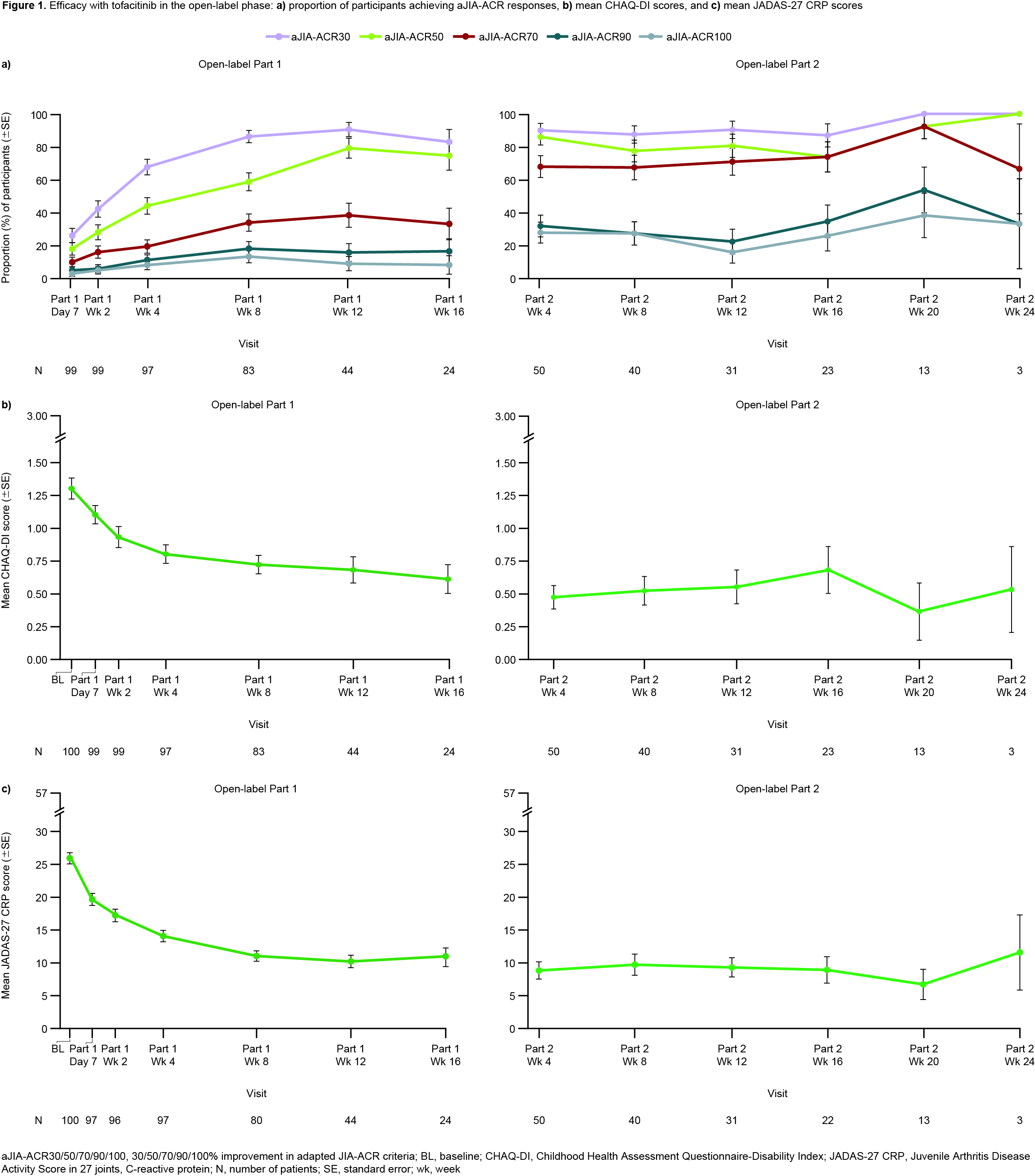
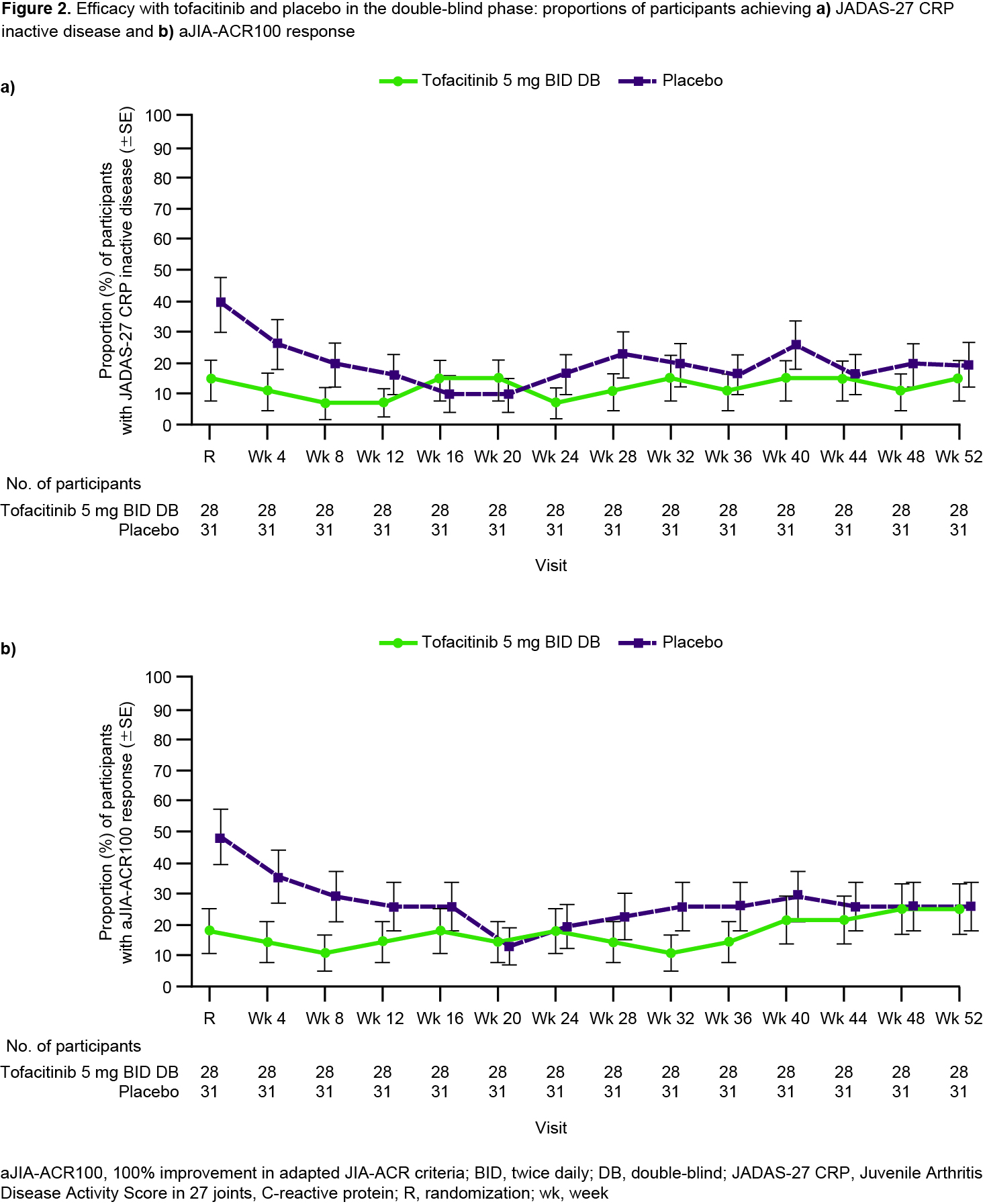
Results: Overall, 100 pts were enrolled in the OL phase from members of the PRINTO/PRCSG networks; 59 were randomized into the DB phase (28 TOF, 31 PBO). The prespecified futility stopping criterion was met and the study did not meet the primary objective of demonstrating statistically significant prolongation in time to sJIA disease flare in the DB phase (HR 0.633; 95% CI 0.296, 1.354; 1-sided p=0.1171) for TOF vs PBO (Table). However, fewer pts experienced flares with TOF (11/28; 39.3%) vs PBO (17/31; 54.8%). Median time to flare was 295 days with PBO and could not be calculated for TOF as < 50% of pts had a flare. During OL Part 1, aJIA-ACR30/70/90 responses were reached by 71/82 (86.6%), 28/82 (34.1%) and 15/82 (18.3%) of pts at wk 8 (Fig 1a). Clinically meaningful improvements in CHAQ-DI (Fig 1b) and JADAS-27 CRP (Fig 1c) were noted during OL Part 1. Responses were generally maintained during OL Part 2 despite CS taper (Fig 1); 38/54 (70.4%) pts achieved tapering criteria. Imbalances in disease activity were observed at DB randomization, including greater proportions of pts with JADAS-27 CRP inactive disease and aJIA-ACR100 responses randomized to PBO compared with TOF (Fig 2). The TOF group generally maintained the efficacy response for these outcomes during the DB phase (Fig 2). In the DB phase, frequencies of treatment‑emergent (TE) adverse events (AEs) were comparable between groups; no pts in the TOF group had severe/serious AEs. A higher percentage of pts had TEAEs leading to discontinuation in the PBO group vs the TOF group, driven by a greater number of sJIA flare TEAEs for PBO (14/31; 45.2%) vs TOF (8/28; 28.6%).
Conclusion: Improvements in sJIA disease activity were observed with OL TOF treatment after 7 days, with >80% of pts achieving an aJIA-ACR30 response by 8 wks. However, the primary efficacy objective in the DB withdrawal phase was not met. Important imbalances at randomization may have influenced the results and been driven by the overall small sample size. Safety findings were consistent with the known profile of TOF in adults and children.
To cite this abstract in AMA style:
Brunner H, Li C, Chinniah K, Uziel Y, Synoverska O, Sawhney S, Calvo Penades I, Louw I, Lu M, Patel P, Weiss P, Chang C, Vranic I, Liu S, Diehl A, Rivas J, Connell C, Koch G, Martini A, Lovell D, Ruperto N. Efficacy and Safety of Tofacitinib in Patients with Active Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-patients-with-active-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-patients-with-active-systemic-juvenile-idiopathic-arthritis/