Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Poorly controlled childhood-onset lupus nephritis (cLN) can lead to end-stage kidney disease (ESKD), requiring kidney replacement therapies with substantial financial and quality of life burden. It is unknown if children with LN reliably receive guideline-concordant care. Here we assessed performance of cLN quality measures, including clinical follow-up every 3 months and laboratory monitoring of nephritis and lupus disease activity at diagnosis and every 3 months.
Methods: We performed a retrospective cohort study of US-based Merative MarketScan Commercial® and 8-State Medicaid Research Databases 2006-2020. Childhood systemic lupus erythematosus (cSLE) was defined as: ≥ 3 outpatient or inpatient visits with SLE International Classification of Diseases (ICD) diagnosis codes, 30-365 days apart, and age 5-18 years at first eligible code. We excluded individuals with: < 6 months of active enrollment in medical and drug coverage prior to first SLE code and any claims for SLE drug use ≥ 6 months before the first SLE code. Among those with cSLE, our approach to identify cLN was: ≥ 2 ambulatory or inpatient visits with ICD diagnosis codes for kidney disease, 30-365 days apart, and occurring 90 days before or any time following first SLE code. We excluded individuals with less than 365 days of enrollment following their first cLN code. We then identified all nephritis monitoring labs (urinalysis, serum creatinine, urine protein to creatinine ratio) and SLE disease activity monitoring labs (complement levels, double-stranded DNA [dsDNA]) using Current Procedural Terminology (CPT) codes. We categorized whether at least one outpatient visit with SLE or LN codes and any laboratory monitoring was completed at least once during each 90-day period in the year following the first LN code.
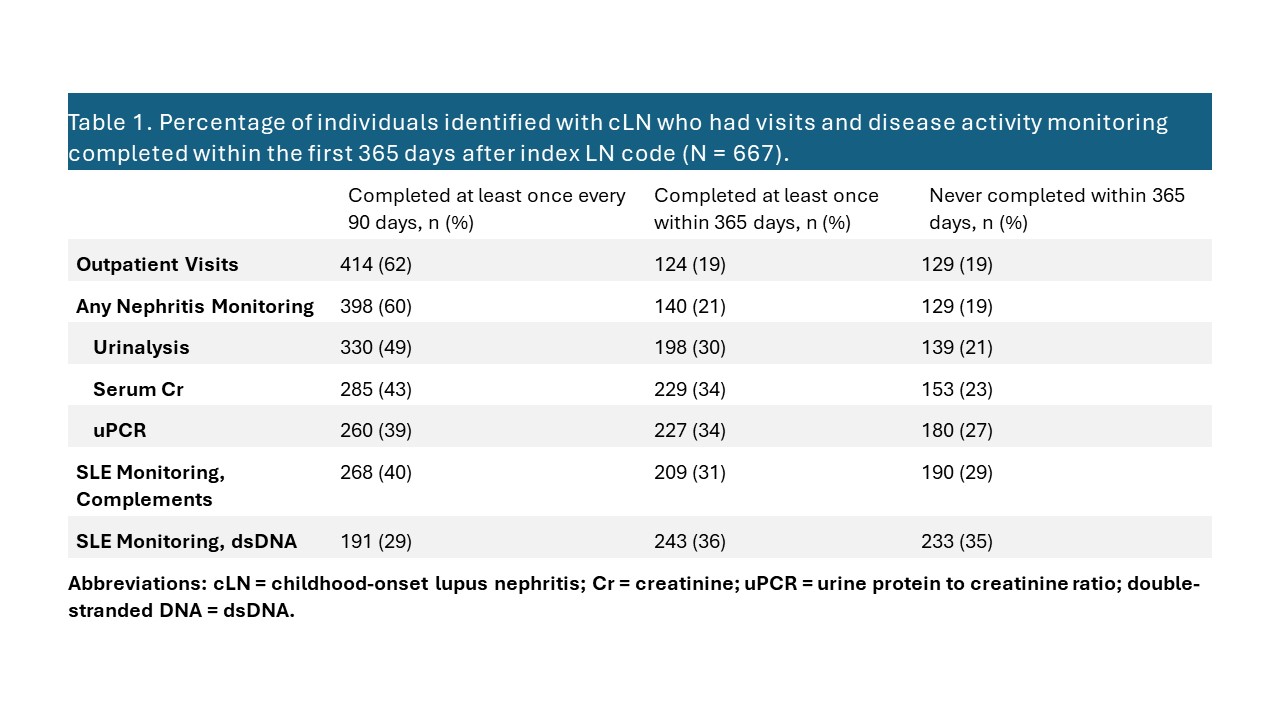
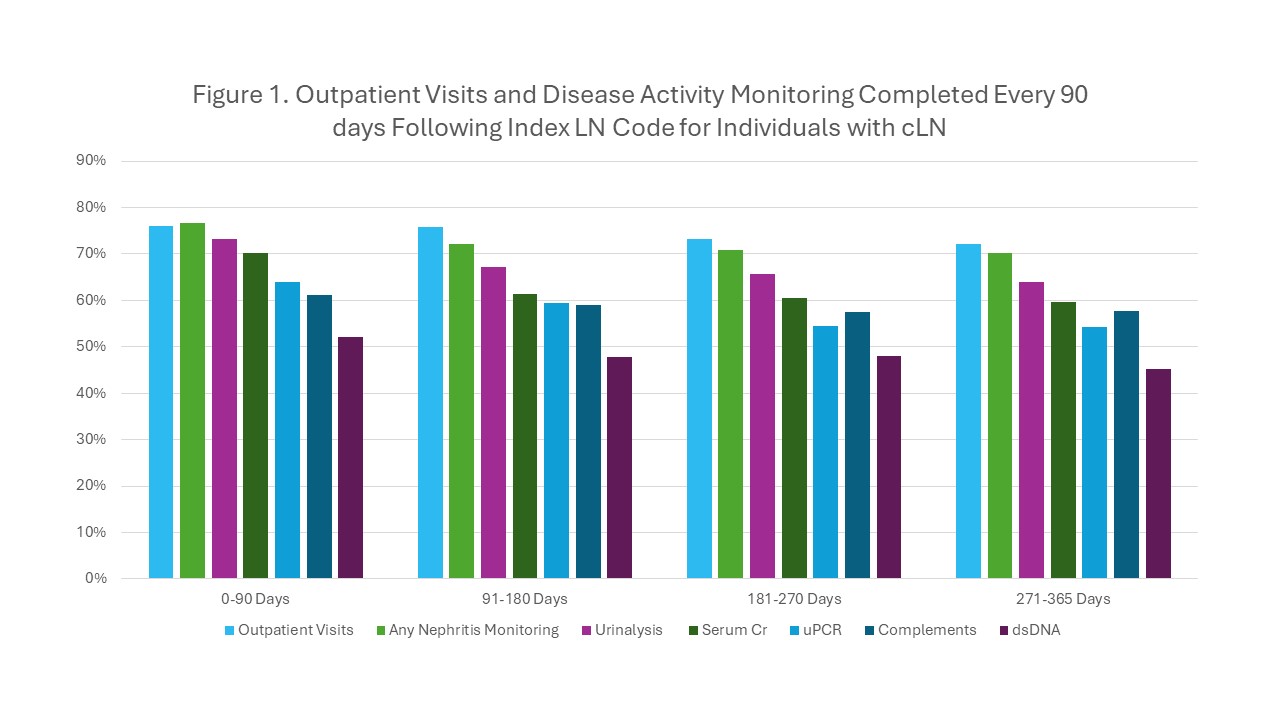
Results: We identified 667 individuals receiving cLN care for at least 365 days from 2006-2020. The majority of cLN patients were female (82%) and commercially-insured (58%), with median (IQR) age at index diagnosis of 15 (13-17) years. During the 365 days following the first LN diagnosis code, 64% of patients completed an outpatient visit at least once every 90 days and 60% completed any LN disease monitoring – 50% urinalysis, 43% serum creatinine, and 39% urine protein to creatinine ratio (Table 1). For SLE disease activity monitoring, 40% completed complement monitoring and 29% completed dsDNA at least once every 90 days. Of note, in the 365 days following the first LN diagnosis code, 19% of individuals completed no LN monitoring, 35% no dsDNA monitoring, and 29% no complement monitoring. For each monitoring type, the percentage of individuals with at least one encounter indicating monitoring within each 90-day period decreased slightly over time (Figure 1).
Conclusion: Using contemporary real-world data for children with cLN, we observed suboptimal completion of cLN disease monitoring, as measured by clinical visits and laboratory assessments, in the year following first LN diagnosis code. Our next step is to analyze the association between appropriate monitoring and development of ESKD. Based on these initial findings, interventions are needed to improve quality of care for patients with cLN.
To cite this abstract in AMA style:
Smitherman E, Leach J, Hersh A, Mannion M, Yazdany J, Curtis J. Quality of Care for Childhood-Onset Lupus Nephritis: Suboptimal Completion of Disease Activity Monitoring [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/quality-of-care-for-childhood-onset-lupus-nephritis-suboptimal-completion-of-disease-activity-monitoring/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quality-of-care-for-childhood-onset-lupus-nephritis-suboptimal-completion-of-disease-activity-monitoring/