Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: CT-P13 SC, a new formulation of infliximab administered subcutaneously, has been approved by European Medicine Agency for the treatment of radiographic axial spondylarthritis (axSpA) and other autoimmune conditions. The main objective of this study was to assess real-world outcomes of CT-P13 SC as a treatment for radiographic and non-radiographic axSpA, in terms of clinical symptoms through physician-completed questionnaires and patient-reported outcome measures (PROMs).
Methods: Data were drawn from the Adelphi Real World axSpA Disease Specific Programme™, a cross-sectional survey with retrospective data collection in France, Germany, Italy, Spain, and the United Kingdom from June 2023 to April 2024. Rheumatologists completed questionnaires regarding patient demographics, clinical status, treatment history, and satisfaction. The same patients completed questionnaires including PROMs and treatment satisfaction. Mean and standard deviation (SD) were reported for continuous variables, analysed using paired t-tests; number and proportion of observations were reported for categorical variables, analysed using McNemar tests. PROMs were analysed descriptively at a single timepoint due to the cross-sectional nature of this study.
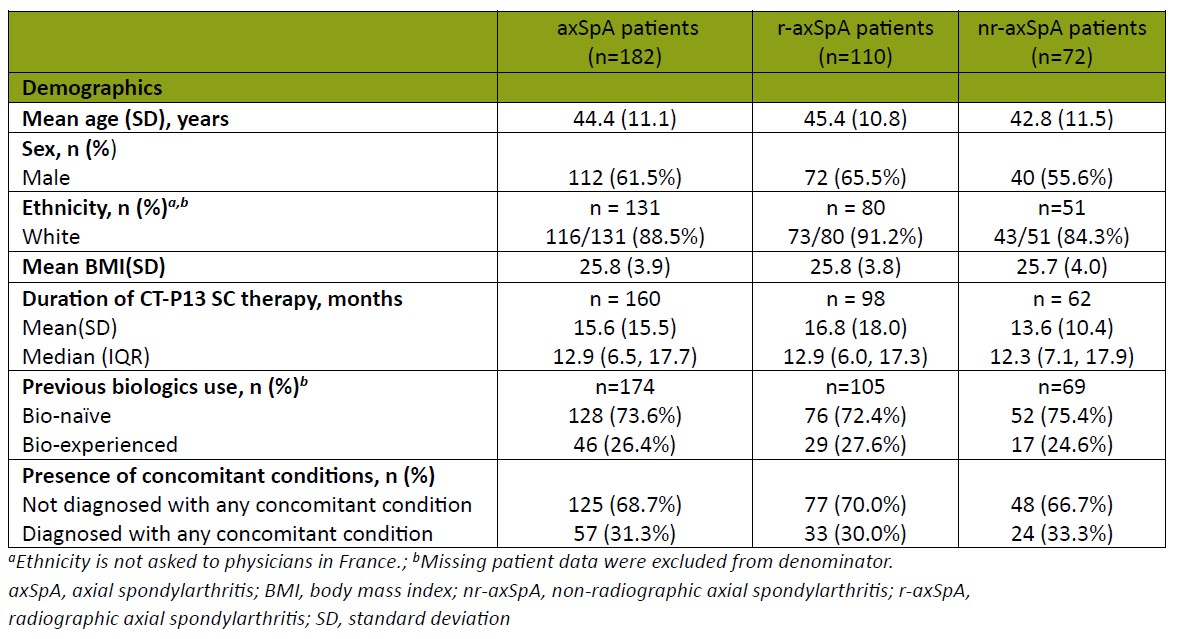
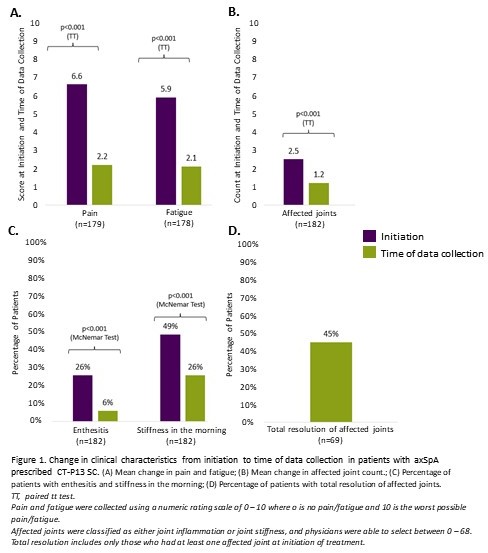
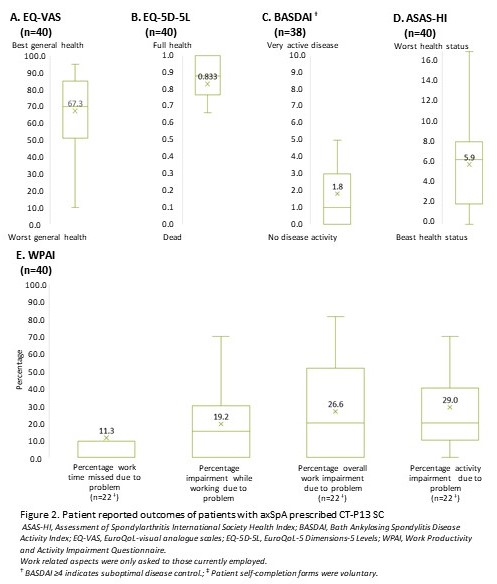
Results: Rheumatologists (n=73) provided data for 182 patients with axSpA. Demographics are reported in Table 1. Patients were treated with CT-P13 SC for a mean (SD) duration of 15.6 (15.5) months. There was a significant decrease in pain (6.6 vs 2.2), fatigue (5.9 vs 2.1), affected joints (2.5 vs 1.2), prevalence of enthesitis (26% vs 6%), morning stiffness (49% vs 26%) between treatment initiation and the time of data collection (all p< 0.001, Figure 1A-C). CT-P13 SC was also associated with a numerical decrease in extra-musculoskeletal manifestations, particularly for uveitis (9% vs 4%, p=0.035). Of patients who had at least one affected joint at treatment initiation , 45% achieved total resolution at data collection (Figure 1D). Patients reported optimal disease control at data collection (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] 1.8, Figure 2). Over 90% of both patients and physicians were satisfied with CT-P13 SC, without any unexpected safety concerns. In subgroup analysis by prior biologic usage, CT-P13 SC demonstrated improvement in severity, fatigue, and BASDAI scores, even in the bio-naïve population which had higher initial disease activity. CT-P13 SC was also effective in treating enthesitis and affected joints with improved BASDAI scores, regardless of concomitant disease.
Conclusion: Patients with active axSpA had significant improvements in clinical outcomes following an average of over 1 year of continuous treatment with CT-P13 SC from treatment initiation. Satisfaction with treatment was high for both patients and physicians, which may be related to good disease control, significant improvements in clinical characteristics, and the lack of safety issues.
axSpA, axial spondylarthritis; BMI, body mass index; nr-axSpA, non-radiographic axial spondylarthritis; r-axSpA, radiographic axial spondylarthritis; SD, standard deviation
Pain and fatigue were collected using a numeric rating scale of 0 – 10 where o is no pain/fatigue and 10 is the worst possible pain/fatigue.
Affected joints were classified as either joint inflammation or joint stiffness, and physicians were able to select between 0 – 68.
Total resolution includes only those who had at least one affected joint at initiation of treatment.
Work related aspects were only asked to those currently employed. † BASDAI ≥4 indicates suboptimal disease control.; ‡ Patient self-completion forms were voluntary.
To cite this abstract in AMA style:
Baraliakos X, Lee Y, Park S, Lee Y, Hughes M, Edwards M, Quiñones E, Sengupta R. A Real-World Study on the Clinical Characteristics and Patient Reported Outcomes of Patients with Active AxSpA Prescribed CT-P13 SC in Five European Countries [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-real-world-study-on-the-clinical-characteristics-and-patient-reported-outcomes-of-patients-with-active-axspa-prescribed-ct-p13-sc-in-five-european-countries/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-real-world-study-on-the-clinical-characteristics-and-patient-reported-outcomes-of-patients-with-active-axspa-prescribed-ct-p13-sc-in-five-european-countries/