Session Information
Date: Monday, November 18, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Identifying patients at risk for developing psoriatic arthritis (PsA) may allow for accelerated diagnosis and treatment and improve long term outcomes for these patients. The Psoriasis Epidemiology Screening Tool (PEST) was developed to identify patients in a dermatology or primary care clinic that should be screened for PsA. We have previously found that the PEST score may be associated with a higher probability for developing PsA. The objective of this study was to examine, in a patient-centered psoriasis registry, the characteristics of patients with a positive PEST screen to those with PsA and those with a negative PEST.
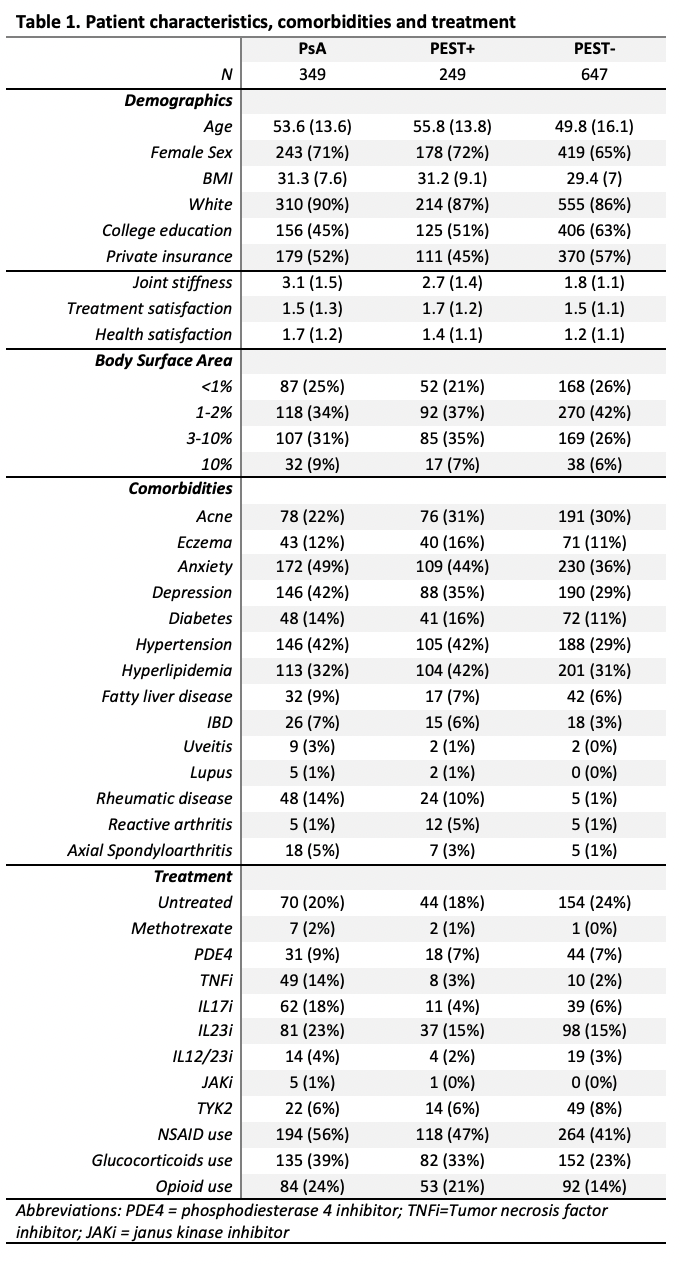
Methods: A cross-sectional study was conducted using baseline data from the Forward Psoriasis Registry, a longitudinal cohort of patients with psoriasis to understand risk for PsA and cardiometabolic disease launched in August 2023. Participants were recruited from US dermatology clinics with dermatologist-diagnosed psoriasis from August 2023 to June 2024. The enrollment questionnaire contains information on psoriasis severity and impact, quality of life, comorbidities, treatment and treatment satisfaction. Participants were age 18 and older and were not required to be on therapy for psoriasis. All variables including diagnosis of PsA were participant reported. We descriptively report demographics, disease characteristics, current treatments, and comorbidities for participants at enrollment by PEST positivity (a score of 3 or more out of 5 items) and PsA diagnosis.
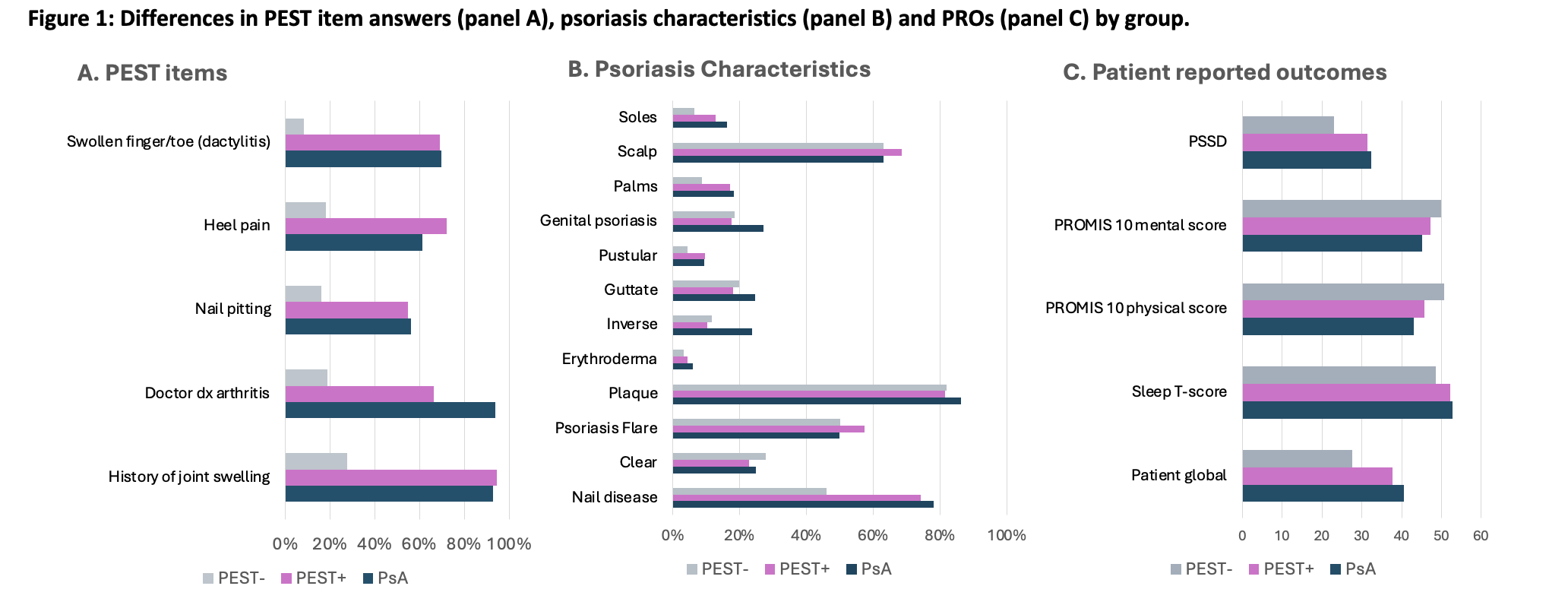
Results: Among 1,269 participants, 1,245 completed a PEST survey and among these 349 (28%) reported a diagnosis of PsA; 249 (20%) had a positive PEST (PEST+) but did not report a diagnosis of PsA, and 647 (52%) had a negative PEST (PEST-) and did not report a PsA diagnosis. Mean age and sex distribution was similar among the groups. Psoriasis characteristics were generally similar although more patients with PsA and PsA-/PEST+ reported nail disease (~75% vs 46% in PEST- group), slightly more moderate to severe psoriasis in the PsA and PEST+ groups, and patients with PsA were more likely to report genital psoriasis and inverse psoriasis. Biologic therapies were more commonly used among patients with PsA compared to those without a PsA diagnosis. A subset of PEST+ participants reported diagnosis of axial spondyloarthritis and reactive arthritis.
Conclusion: Among participants in this prospective psoriasis registry, those who were PEST+ were similar to those with a diagnosis of PsA in terms of psoriasis characteristics and comorbidities. PEST may serve as a proxy for PsA or PsA risk in longitudinal cohort studies, however some patients may have competing diagnoses or other forms of arthritis. The use of PEST as a predictor of PsA will be prospectively tested in this cohort.
To cite this abstract in AMA style:
Ogdie A, Song W, Gelfand J, Lonowski S, Schumacher R, Michaud K. Patients with Psoriasis with a Positive Psoriasis Epidemiology Screening Tool (PEST): Similarities and Differences Compared to PsA and Patients with Psoriasis and a Negative PEST [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/patients-with-psoriasis-with-a-positive-psoriasis-epidemiology-screening-tool-pest-similarities-and-differences-compared-to-psa-and-patients-with-psoriasis-and-a-negative-pest/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patients-with-psoriasis-with-a-positive-psoriasis-epidemiology-screening-tool-pest-similarities-and-differences-compared-to-psa-and-patients-with-psoriasis-and-a-negative-pest/