Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Tofacitinib is the first Janus Kinase Inhibitor (JAKi) approved for treating adults with rheumatoid arthritis (RA). Recently, its use has been linked to an increased risk of major adverse cardiovascular events (MACE) and malignancy. These concerns emerged from the findings of the oral surveillance trial, which is a mandated post-authorization safety trial designed to assess the risk of MACE and malignancy in JAKi versus Tumor Necrosis Factor inhibitors (TNFi) patients (1). We aimed to investigate whether these concerns have been replicated in real world settings. Our objective was to quantify the risk of MACE, overall malignancies, and non-melanoma skin cancer (NMSC) associated with the use of JAKi versus TNFi in RA patients in daily clinical practice settings.
Methods: A systematic literature review was conducted using four databases (Medline, Embase, Scopus and Web of Science). Databases were searched from inception until March 30th, 2024. Only observational cohort studies that assessed the risk of MACE and malignancy in JAKi versus TNFi in adult RA patients were retrieved. A quantitative data synthesis was carried out using a random effects model with the inverse variance method to calculate the pooled hazard ratios (HR) with 95% confidence interval (CI). Risk of bias in eligible studies was assessed using Risk of Bias In Non-randomized Studies – of Interventions (ROBINS-1) tool created by the Cochrane collaboration (2). This review was registered with the International Prospective Register of Systematic Reviews (PROSPERO registration ID: CRD42024525148).
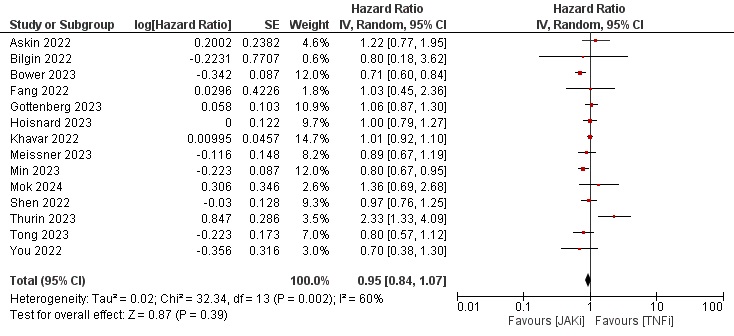
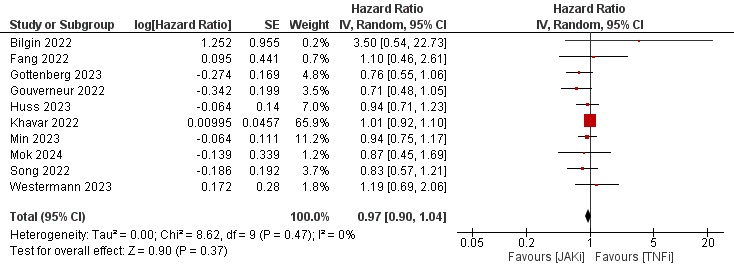
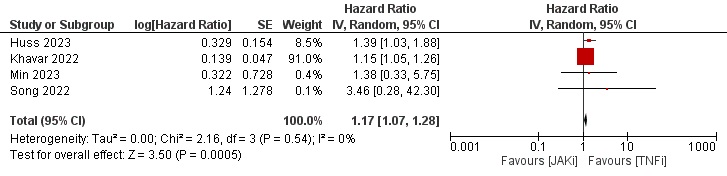
Results: Thirty-five studies describing a total of more than 350,000 patients were eligible for our study. Most patients were female and above 50 years of age on average. Geographically, 14 studies were from Asia, 13 from Europe, 7 from North America, and 1 from Oceania. Tofacitinib was evaluated solely against TNFi in 14 studies. Baricitinib and Upadacitinib were included in 16 and 6 studies, respectively. Around half of the studies were judged as low risk of bias based on the ROBINS-I tool. The results of data synthesis showed a HR of 0.95 [0.84–1.07], 0.97 [0.90–1.04], and 1.17 [1.07–1.28] (Figures 1,2, and 3, respectively) for the risk of MACE, malignancy and NMSC in JAKi versus TNFi patients.
Conclusion: JAKi were not associated with increased risk of MACE or malignancy when compared to TNFi in a real-world setting. However, JAKi were associated with a small increased risk of NMSC.
References
1. R. YS, L. BD, R. MT, G. KG, Roy F, L. RJ, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med [Internet]. 2022 Jan 26;386(4):316–26. Available from: https://doi.org/10.1056/NEJMoa2109927
2. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ [Internet]. 2016 Oct 12;355:i4919. Available from: http://www.bmj.com/content/355/bmj.i4919.abstract
To cite this abstract in AMA style:
Haider M, Alalwan O, Harris H. The Risk of Malignancy and Major Adverse Cardiovascular Events with the Use of Janus Kinase Inhibitors in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Real-World Evidence [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-risk-of-malignancy-and-major-adverse-cardiovascular-events-with-the-use-of-janus-kinase-inhibitors-in-patients-with-rheumatoid-arthritis-a-systematic-review-and-meta-analysis-of-real-world-eviden/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-risk-of-malignancy-and-major-adverse-cardiovascular-events-with-the-use-of-janus-kinase-inhibitors-in-patients-with-rheumatoid-arthritis-a-systematic-review-and-meta-analysis-of-real-world-eviden/