Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Idiopathic recurrent pericarditis (IRP) is a rare autoinflammatory syndrome marked by recurrent pericardial inflammation after an initial episode of acute pericarditis. Standard treatment involves colchicine and aspirin/NSAIDs, with corticosteroids as second-line therapy and immunosuppressive drugs, including targeted therapies like anakinra, as third-line options. Anakinra, an interleukin-1 inhibitor, has shown promise in treating IRP. This study aimed to evaluate the safety and efficacy of anakinra in real-life settings.
Methods: Patients with IRP who received anakinra from 2018 to 2024 in Midi-Pyrénées, France, were retrospectively identified. They met the 2015 ESC criteria for recurrent pericarditis. Demographic data, clinical features, and treatment responses were analyzed. A complete response was defined as the absence of symptoms, elevated C-reactive protein, and pericardial effusion. Event-free survival (EFS) was estimated using the Kaplan-Meier method. The glucocorticoid-sparing effect was evaluated with Fisher’s exact test, with significance set at 0.05.
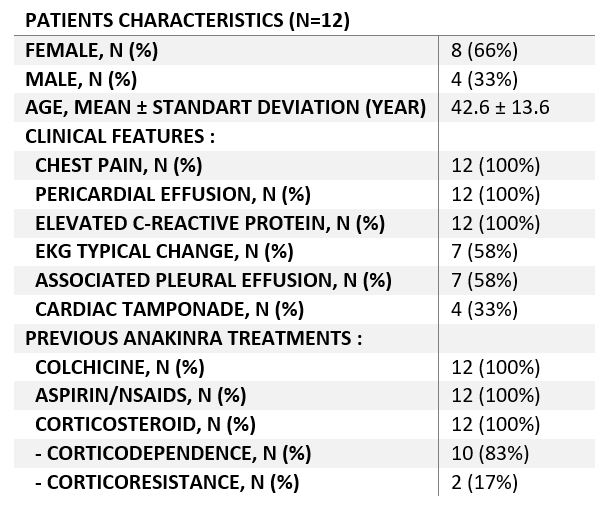
Results: The study enrolled 12 patients, with 4 (33%) males and 8 females. The mean age at diagnosis was 42.6 ± 13.6 years. All patients presented with chest pain, elevated C-reactive protein levels, and pericardial effusion. Characteristic ECG changes were noted in 7 patients (58%). Additionally, 7 patients (58%) had associated pleural effusion, and 4 (33%) experienced cardiac tamponade. Initial treatment consisted of colchicine combined with aspirin or NSAIDs as first-line therapy, followed by corticosteroids as second-line therapy. Surgical pericardial drainage was performed in 6 patients (50%).
Among the participants, 10 (83%) were corticosteroid-dependent, and 2(16.6%) were corticosteroid-resistant. Patients had an average of 5.5 ± 1.4 pericarditis attacks before starting anakinra, with a mean duration from the first attack to anakinra initiation of 15.4 ± 13.9 months.
Anakinra was administered daily via subcutaneous injection (100 mg/day) to all patients. Ten patients (83.3%) received concomitant colchicine with anakinra. The mean duration of anakinra treatment was 28.4 ± 21.3 months. During anakinra therapy, daily corticosteroid use significantly decreased from 17.9 ± 10.68 mg to 0 mg (p < 0.005). A complete response was observed in all patients (100%).
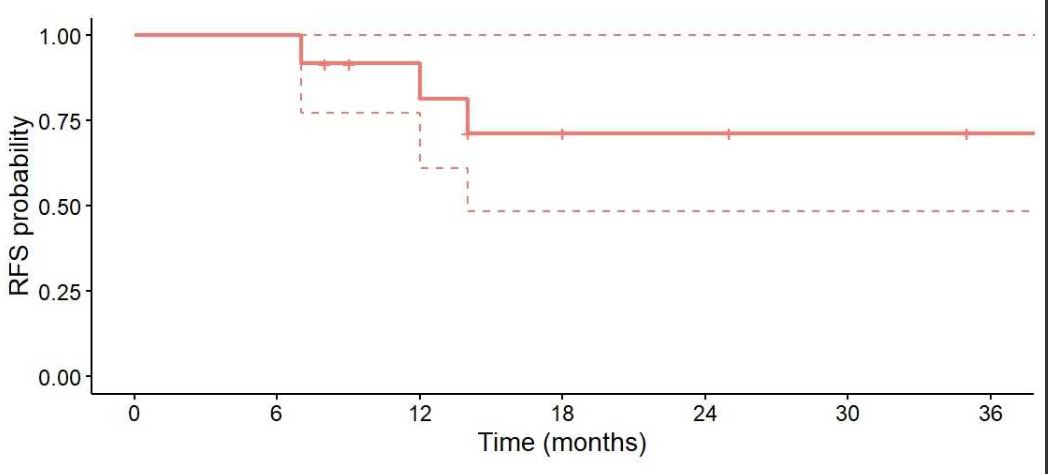
An attempt to discontinue anakinra was made in 10 patients (83.3%): 3 patients experienced pericarditis relapse, and 3 successfully discontinued anakinra treatment. All relapses were managed by reintroducing daily 100 mg anakinra injections. The event-free survival rate at 18 months of anakinra use was 75%. No serious adverse events were reported during follow-up.
Conclusion: Anakinra is a safe and effective treatment for patients with idiopathic recurrent pericarditis resistant to conventional therapies. It significantly reduced the frequency of pericarditis attacks and enabled the discontinuation of corticosteroids in most patients. These findings support anakinra as a valuable third-line therapy for managing IRP.
To cite this abstract in AMA style:
Michaud M, Sailler L, LEMEU M, CASTEL B, Vacheret F, Prudhomme L, Pugnet G. Effective Management of Idiopathic Recurrent Pericarditis with Anakinra in Clinical Practice [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effective-management-of-idiopathic-recurrent-pericarditis-with-anakinra-in-clinical-practice/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effective-management-of-idiopathic-recurrent-pericarditis-with-anakinra-in-clinical-practice/