Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The management of immune-mediated adverse events (irAEs) during treatment with immune checkpoint inhibitors (ICIs) involves high dosages of glucocorticoids (GCs).
Despite being effective in suppressing inflammation, GCs carry many risks including the facilitation of cancer progression and counteracting the benefits of ICIs.
This study aimed to investigate the efficacy and safety of methotrexate (MTX), as a GC-sparing agent, in arthritides induced by ICIs.
Methods: During 2023, all the adult patients who developed ICI-induced arthritis were assessed. Individuals who developed at least one episode of clinical synovitis were included.
Disease activity was assessed by the activity score on 28 joints by C-reactive protein (DAS28-CRP).
Patients were followed-up every 3 months in line with a tight control approach. MTX was prescribed to all patients.
Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was used to describe the oncologic outcomes of the patients at first year.
Results: We evaluated 16 patients (M/F = 6/10, mean age 63.4 years) affected by melanoma (62.5%), colo-rectal cancer (25%) and lung cancer (12.5%) receiving anti-PD1 (pembrolizumab in 33%, nivolumab in 33%, cemiplimab in 7%, nivolumab + pembrolizumab in 7%), PDL1 (atezolizumab in 13%) and/or CTLA4 blockers (ipilimumab + nivolumab in 7%).
Polyarthritis occurred in 56% of patients, oligo-arthritis in 37% and a polymyalgia rheumatica-like syndrome in 7% after a mean onset time of 60 ± 37 days from the starting of ICI.
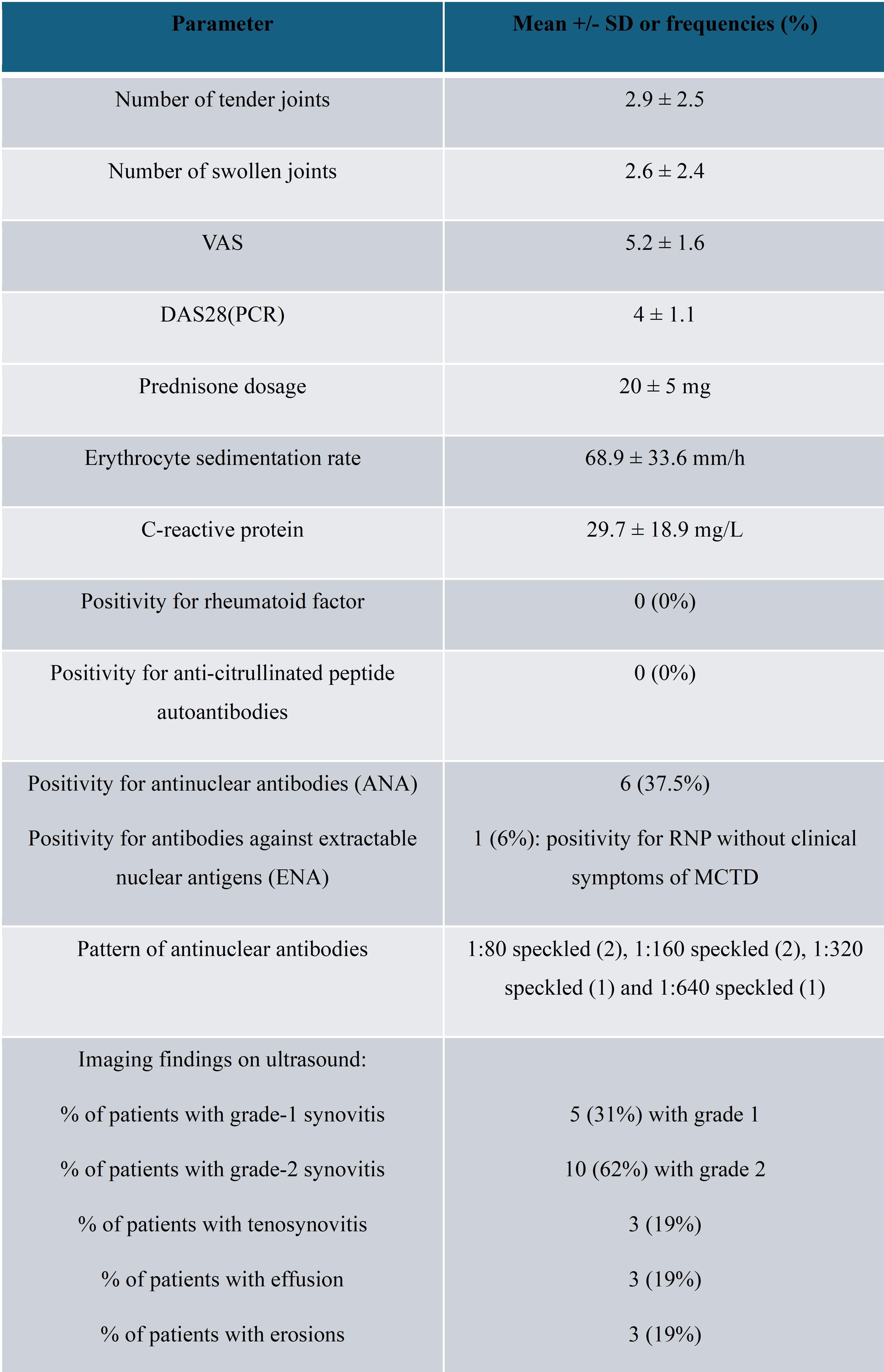
The clinical features of patients at first visit (V0) are reported in Table 1. MTX was prescribed with a mean dosage of 8.3 ± 2.25 mg/weekly; in more than half of them (57%) it was started at the V0.
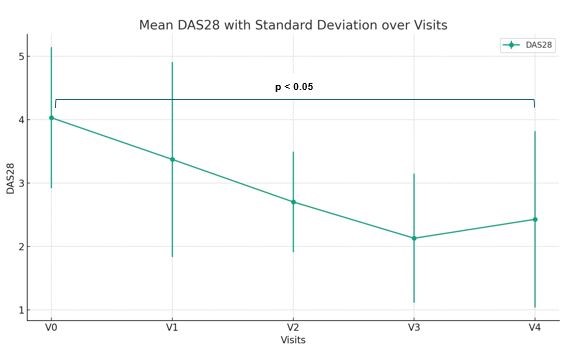
DAS28-CRP values improved during the follow-up (all p < 0.05 for each value compared to V0, Fig 1). All the patients achieved disease remission and significantly reduced prednisone dosage at first year (5 ± 2.5 mg at V4, p < 0.001 compared to V0).
No major toxicities related to MTX were observed. One patient exhibited mild hyper-transaminasemia, with liver enzyme levels rising to less than 1.5 times the upper limit.
Ten patients (62.5%) maintained the complete cancer response at first year whereas the other 6 (37.5%) had a stable disease. No one displayed cancer progression.
Conclusion: Initiating MTX early led to effectively managing ICI-induced arthritis by progressively reducing the GC dosage while maintaining a favorable safety profile both in terms of pharmacological tolerance and oncologic safety at first year.
This approach successfully attained a target of less than 10 mg of prednisone daily, which is recommended
to improve overall survival in these patients [1].
References: [1] Kostine et al. Ann Rheum Dis 2021.
To cite this abstract in AMA style:
Hysa E, Casabella A, Iandolino N, Gotelli E, Genova C, Tanda E, Pizzorni C, smith v, Sulli A, Cutolo M, Paolino S. Efficacy and Safety of Methotrexate in Immune Checkpoint Inhibitor-induced Arthritis: A Retrospective Longitudinal Monocentric Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-methotrexate-in-immune-checkpoint-inhibitor-induced-arthritis-a-retrospective-longitudinal-monocentric-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-methotrexate-in-immune-checkpoint-inhibitor-induced-arthritis-a-retrospective-longitudinal-monocentric-study/