Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Pregnant women are particularly at risk of severe Coronavirus disease 2019 (COVID-19) infection, which is why the Centers for Disease Control and Prevention (CDC) recommends vaccination for pregnant women to prevent severe disease. Despite this recommendation, the frequency of adverse reactions to COVID-19 vaccination in pregnant patients with autoimmune inflammatory rheumatic diseases (AIIRD) remains unknown. We investigated the frequency of short-term (≤ 7 days) adverse reactions following COVID-19 vaccination in pregnant women with AIIRD.
Methods: A descriptive analysis of the incidence of side effects following COVID-19 vaccination in pregnant women with AIIRD was conducted using data from the MotherToBaby Study (MTB) registry, a prospective cohort study designed to assess the safety of drug and vaccine exposure during pregnancy. This study enrolled pregnant women living in the United States or Canada between January 2021 and September 2022.
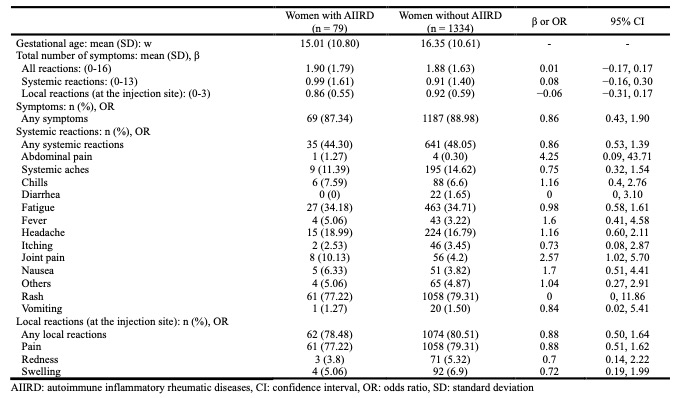
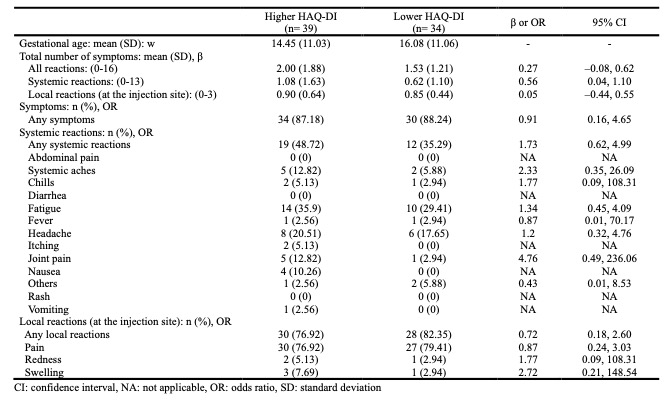
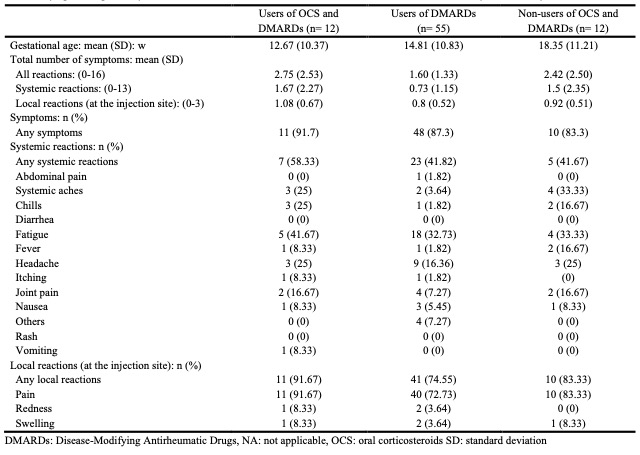
Results: Of 1413 participants who received dose 1 of the COVID-19 vaccine during pregnancy, 79 had AIIRD. There was no significant difference in the total number of adverse reactions between pregnant women with and without AIIRD (β = –0.01, 95% CI: –0.17, 0.17). Pregnant women with higher HAQ-DI in AIIRD had a significantly higher total number of systemic reactions (β = 0.56, 95% CI: 0.04, 1.10) than those with lower HAQ-DI, although the total number of adverse reactions was not significantly different (β = 0.27, 95% CI: –0.08, 0.62). Users of DMARDs alone tended to experience fewer adverse reactions compared to those using both OCS and DMARDs or neither. (All reactions: mean (SD) of users of OCS and DMARDs = 2.75 (2.53), mean (SD) of users of DMARDs alone = 1.60 (1.33), mean (SD) of none users = 2.42 (2.50)).
Conclusion: We found no significant difference in the frequency of COVID-19 vaccine-related side effects between pregnant women with and without AIIRD. Patients with AIIRD who have higher levels of functional impairment may have a slightly higher frequency of short-term adverse effects. The results of this assessment may help provide information for pregnant women with AIIRD regarding COVID-19 vaccination.
To cite this abstract in AMA style:
Kaneshita S, Chambers C, Johnson D, Kavanaugh A, Garfein R, Bandoli G. Analysis of Short-Term Side Effects Following COVID-19 Vaccination in Pregnancies Complicated by Autoimmune Inflammatory Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/analysis-of-short-term-side-effects-following-covid-19-vaccination-in-pregnancies-complicated-by-autoimmune-inflammatory-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-short-term-side-effects-following-covid-19-vaccination-in-pregnancies-complicated-by-autoimmune-inflammatory-rheumatic-diseases/