Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: CC-99677 (also known as BMS-986371) is a novel, orally bioavailable, small-molecule covalent inhibitor of mitogen-activated protein (MAP) kinase-activated protein kinase 2 (MK2). The MK2 enzyme is regulated through direct phosphorylation by p38 MAP kinase and it enhances stabilization and translation of mRNA of proinflammatory cytokines, such as TNF-α, IL-17 and IL-6. This is accomplished by phosphorylation and inhibition of the mRNA destabilizing function of tristetraprolin, which leads to cytokine translation. CC-99677 is a potent and selective covalent inhibitor of MK2 in biochemical and cellular assays. We aimed to evaluate the dose-dependent efficacy of oral CC-99677 compared to placebo in subjects with ankylosing spondylitis (AS) and inadequate response to NSAIDs.
Methods: This was a Phase 2, multicenter, randomized, double-blind, placebo-controlled, parallel-group, efficacy and safety study to assess response to CC-99677 treatment by measuring signs and symptoms of ankylosing spondylitis, objective measures of disease activity, quality of life assessments, safety, and tolerability over a 12-week double-blind period. This study also aimed to assess the efficacy and long-term safety of CC-99677 in a 52-Week Long-term Extension Period. Patients were randomized 1:1:1 to CC-99677 150 mg PO QD, CC-99677 60 mg PO QD, or matching placebo, stratified by screening hsCRP concentration (≤ or >5.0mg/L). Main inclusion criteria were a diagnosis of AS, fulfilment of the modified New York criteria for AS, active symptoms (BADSDAI >4 and Total Back Pain (TBP) >4) despite ≥2 NSAID, and naïve to bioDMARD in the main study. A total of 147 subjects were planned to be randomized, 49 subjects to each treatment group, for 80% power to detect a 25% difference in ASAS20 response at Week 12, the primary endpoint, between either active treatment group and placebo. The Hochberg procedure was applied to the analysis to adjust for multiplicity. A substudy planned recruitment up to 50 patients that were bioDMARD-inadequate responders (IR).
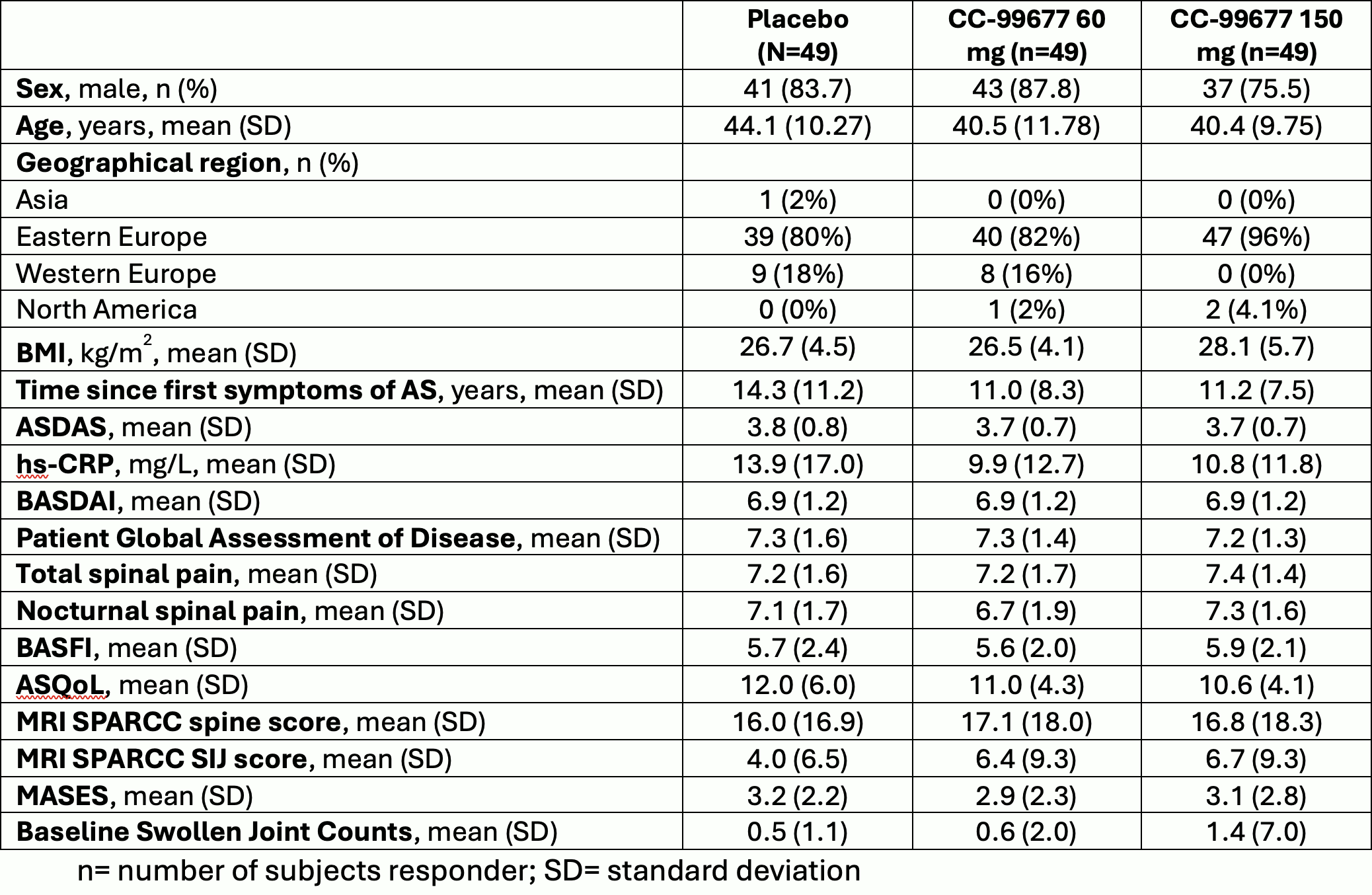
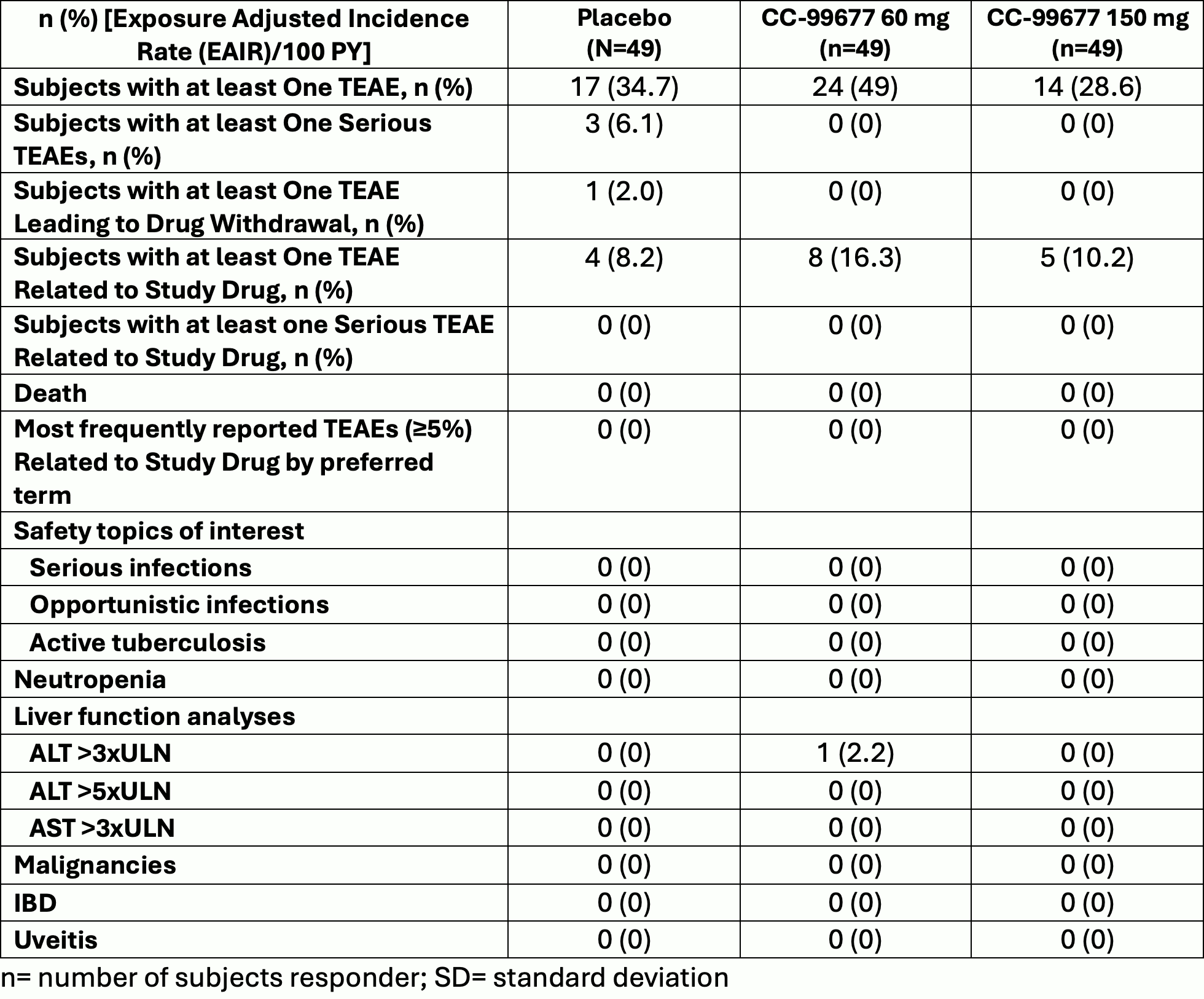
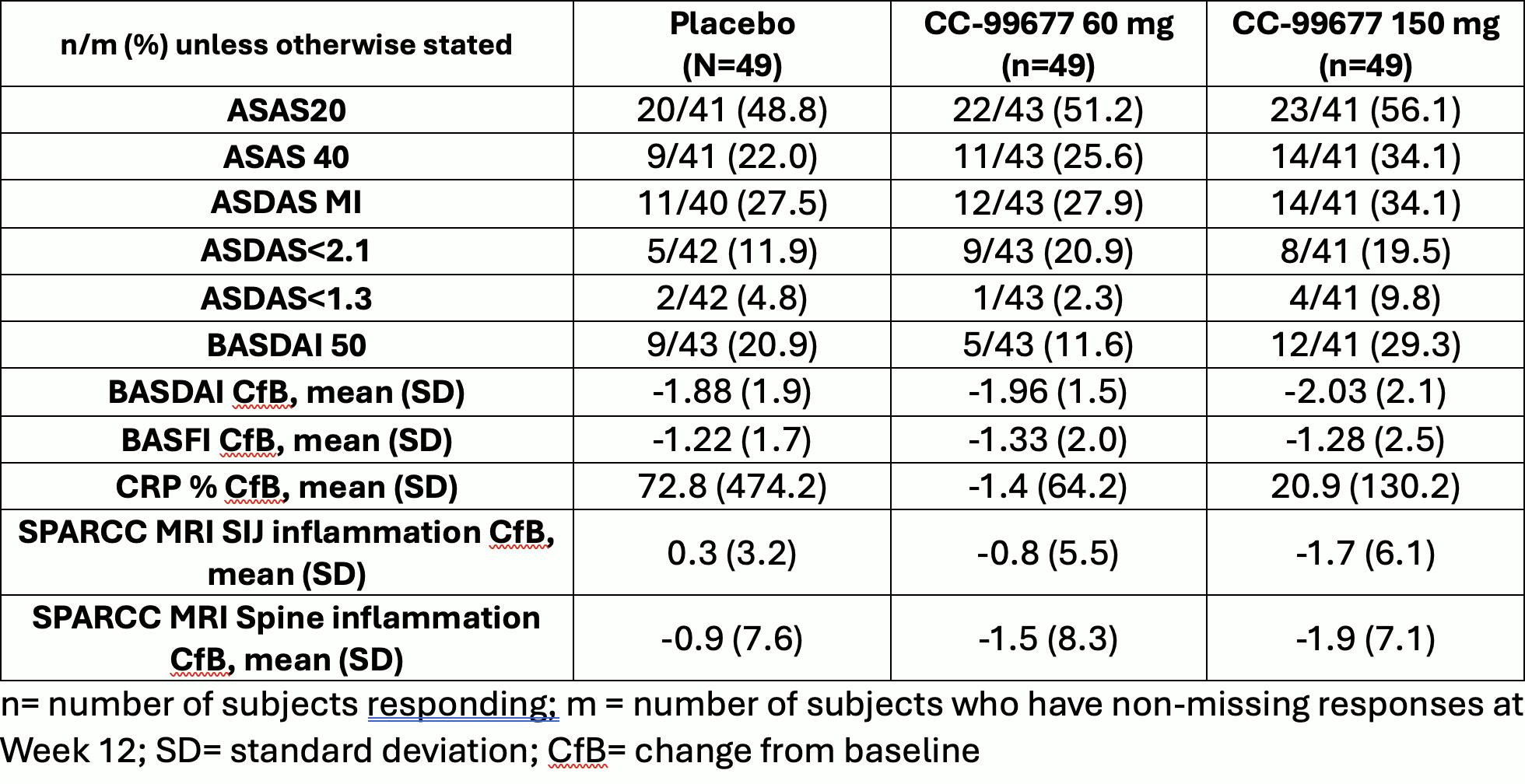
Results: We present results only for the biologic-naïve main study. 147 subjects were enrolled: 49 in CC-99677 60 mg group, 49 in CC-99677 150 mg group, 49 subjects in placebo. A total of 123 (83.7%) subjects completed treatment in the double-blind, 12-week placebo-controlled period and 24 (16.3%) subjects discontinued treatment. The main reason for discontinuation was study termination by Sponsor Steering Committee after interim analysis when approximately 50 subjects in the biologic-naïve main study completed 12 weeks of treatment. Baseline characteristics were typical of an AS trial population (Table 1). Treatment with CC-99677 was generally safe and well-tolerated (Table 2). For efficacy, ASAS20 and ASAS 40 at Week 12 was 51.2% and 25.6% in the CC-99677 60 mg group, 56.1% and 34.1% in the 150 mg group, and 48.8% and 22.0% in the placebo group. No significant group differences were noted for key secondary endpoints at week 12 (ASDAS, BASDAI, BASFI, SPARCC MRI SIJ and spine scores) (Table 3).
Conclusion: The study was prematurely terminated for futility. The termination was not related to any observed adverse events, or perceived safety findings associated with CC-99677.
To cite this abstract in AMA style:
Maksymowych W, Lambert R, Śliwinska-Stańczyk P, Klimiuk P, Yeshokumar A, Cerullo E, Kepich R, Pachai C, Greenberg S. A Phase 2 Multicenter, Randomized, Double-Blinded, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of a Mitogen-activated Protein Kinase-Activated Protein Kinase 2 (MK2) Inhibitor in Active Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-phase-2-multicenter-randomized-double-blinded-placebo-controlled-parallel-group-study-to-evaluate-the-efficacy-and-safety-of-a-mitogen-activated-protein-kinase-activated-protein-kinase-2-mk2-i/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2-multicenter-randomized-double-blinded-placebo-controlled-parallel-group-study-to-evaluate-the-efficacy-and-safety-of-a-mitogen-activated-protein-kinase-activated-protein-kinase-2-mk2-i/