Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: CD19 is an appealing therapeutic target due to its ubiquitous expression on B cells and plasmablasts, which play a key role in the pathogenesis of autoimmune diseases (ADs) with high unmet need.1,2 CC-97540 (BMS-986353) is an investigational CD19-targeted CAR T cell therapy that expresses the CD19 CAR used in FDA-approved lisocabtagene maraleucel (liso-cel), but is manufactured using the NEX-TTM process, to shorten manufacturing time, improve potency, and optimize phenotypic attributes of the CAR T cell product. Here we report preliminary data of CC-97540 in severe, refractory ADs.
Methods: This ongoing phase 1, multicenter, open-label study assesses safety (primary endpoint), efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) in patients (pts) with severe, refractory ADs (NCT05869955). Before leukapheresis, pts were tapered off all therapy. After leukapheresis, pts T cells were purified and engineered with the NEX-T manufacturing process to express the liso-cel CAR construct. Pts received a single infusion of CC-97540 2–7 days after lymphodepleting chemotherapy (3 days fludarabine [30 mg/m2], cyclophosphamide [300 mg/m2]). In the dose-escalation phase, pts were treated with 10 × 106 or 25 × 106 CAR+ T cells.
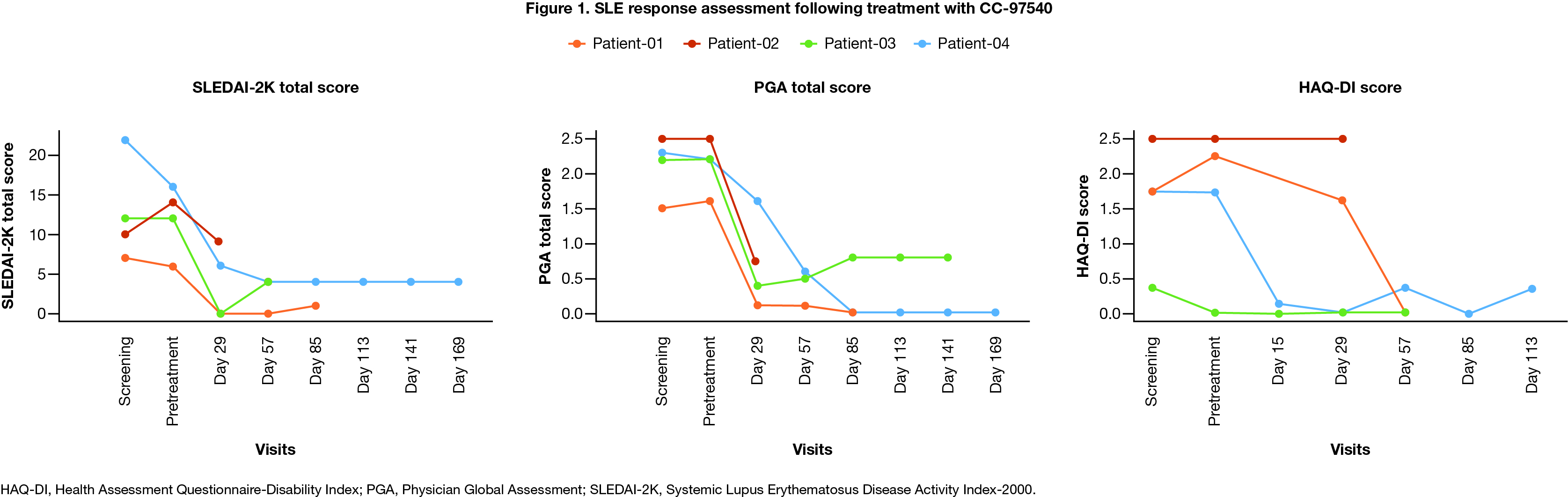
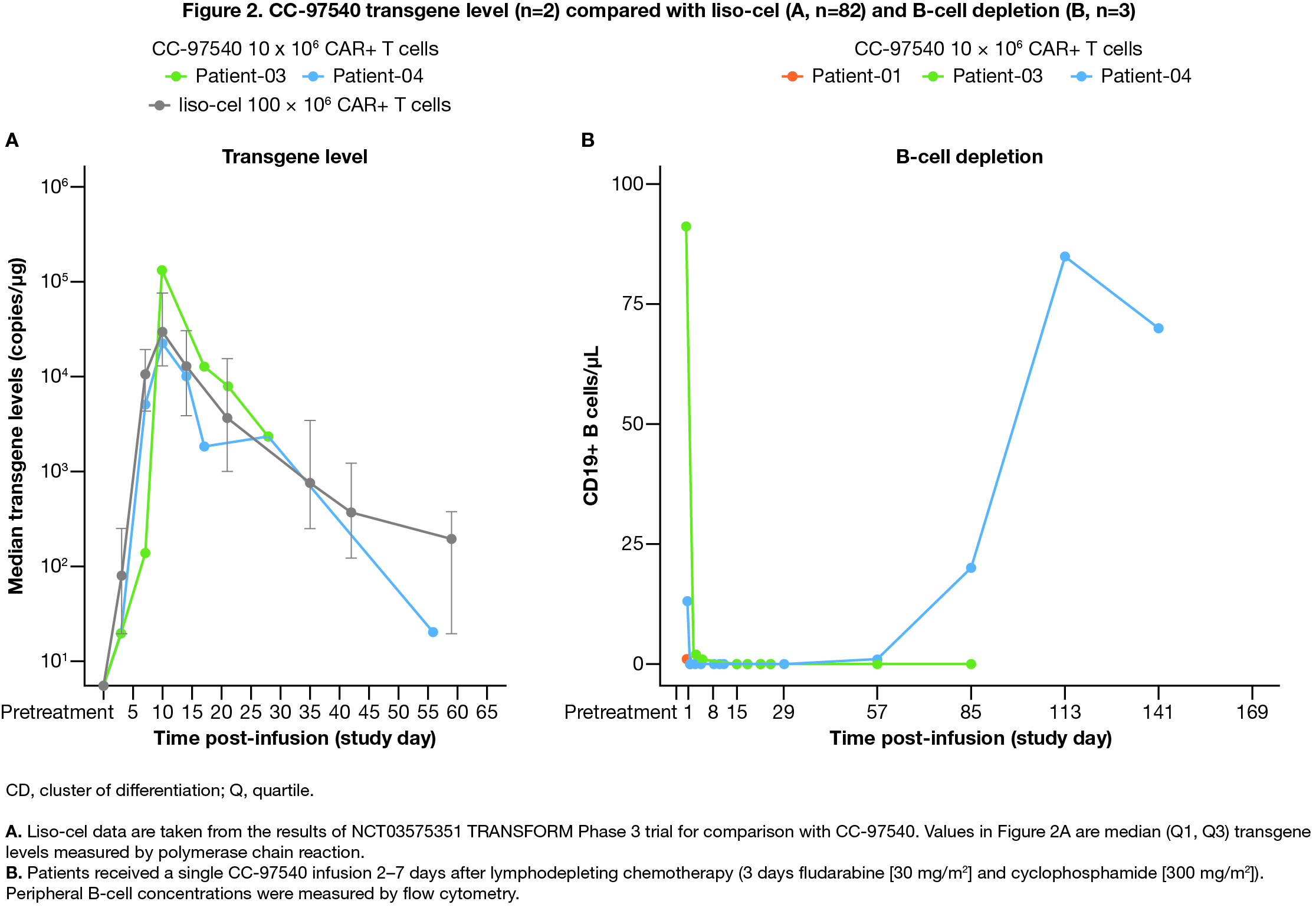
Results: As of Jun 5, 2024, 9 pts have been enrolled; 4 were treated with CC-97540. All 4 pts with SLE entered the study with severe renal organ system involvement (BILAG category A) and had disease refractory to multiple prior therapies (range 5-8). Grade 1 cytokine release syndrome was reported in 1 pt, related to CC-97450, and resolved in 1 day without anti-cytokine therapy or corticosteroids. Grade 3 or 4 transient lymphodepletion-related cytopenia was observed in 3/4 (75%) pts. No neurotoxicity, SAE, or DLT was reported. Despite discontinuation of lupus therapy, all pts had significant improvement in SLEDAI score, PGA, and HAQ-DI (Fig 1). Most SLEDAI domains resolved to 0 except proteinuria >0.5 g/24 hr in 3 pts, in whom reduction in proteinuria was observed. All pts remain off therapy without evidence of new disease activity. dsDNA seroconverted and complement normalized for all pts with abnormal values. Transgene PK analysis demonstrated similar expansion to liso-cel at a 10-fold lower dose (Fig 2A) and PD analysis demonstrated complete B-cell depletion (Fig 2B). After CC-97540 depletion, repopulated B cells were predominantly naive with a marked reduction in plasmablasts. Despite B cell recovery, one pt had durable clinical response without evidence of new disease activity.
Conclusion: Results from first pts indicate promising preliminary safety and efficacy of CC-97540 at low doses. The data also show robust CAR T cell expansion and complete B cell depletion. CC-97540, a NEX-T investigational CD19 CAR T cell product, is a more potent cellular drug product than liso-cel and can be manufactured with a more rapid processing time and greater capacity. Updated safety, efficacy, and translational data will be presented.
1. Szelinski F, et al. Curr Opin Rheumatol 2022;34(2):125-132.
2. Müller F, et al. N Engl J Med 2024;390:687-700
Acknowledgements: Steven Tretyakov (Caudex) PharmD, funded by Bristol Myers Squibb; Alexis Melton, Naomey Sarkis, Alisha Desai
To cite this abstract in AMA style:
Schett G, Littlejohn E, Kramer N, Saxena A, Mease P, Wiesendanger M, Müller F, Reshef R, Caimi P, Cherry M, Hsu J, Patel K, Azzi J, Falcon S, Ly T, Ogasawara K, Das S, Thorpe J, Maldonado M, Stifano G, Koegel A, Askanase A. A Phase 1, Multicenter, Open-Label Study to Establish the Preliminary Tolerability, Efficacy, Pharmacokinetics, and Pharmacodynamics of CC-97540 (BMS-986353), a CD19-directed CAR T Cell Therapy Manufactured Using a Next-generation Process, for Severe, Refractory Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-phase-1-multicenter-open-label-study-to-establish-the-preliminary-tolerability-efficacy-pharmacokinetics-and-pharmacodynamics-of-cc-97540-bms-986353-a-cd19-directed-car-t-cell-therapy-manufa/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-1-multicenter-open-label-study-to-establish-the-preliminary-tolerability-efficacy-pharmacokinetics-and-pharmacodynamics-of-cc-97540-bms-986353-a-cd19-directed-car-t-cell-therapy-manufa/