Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) inhibitors are under investigation in chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis. Ocadusertib (LY3871801; R552) is a potent and selective allosteric inhibitor of RIPK1 that blocks inflammatory cell death and is currently being studied in clinical trials. Here, we present preclinical and Phase 1 clinical data on ocadusertib.
Methods: Preclinical data were generated in house using standard methods. A first-in-human (FIH) Phase 1 study (EudraCT: 2019-002520-32) evaluated the safety, tolerability, and PK of various formulations of ocadusertib in healthy adult subjects.1
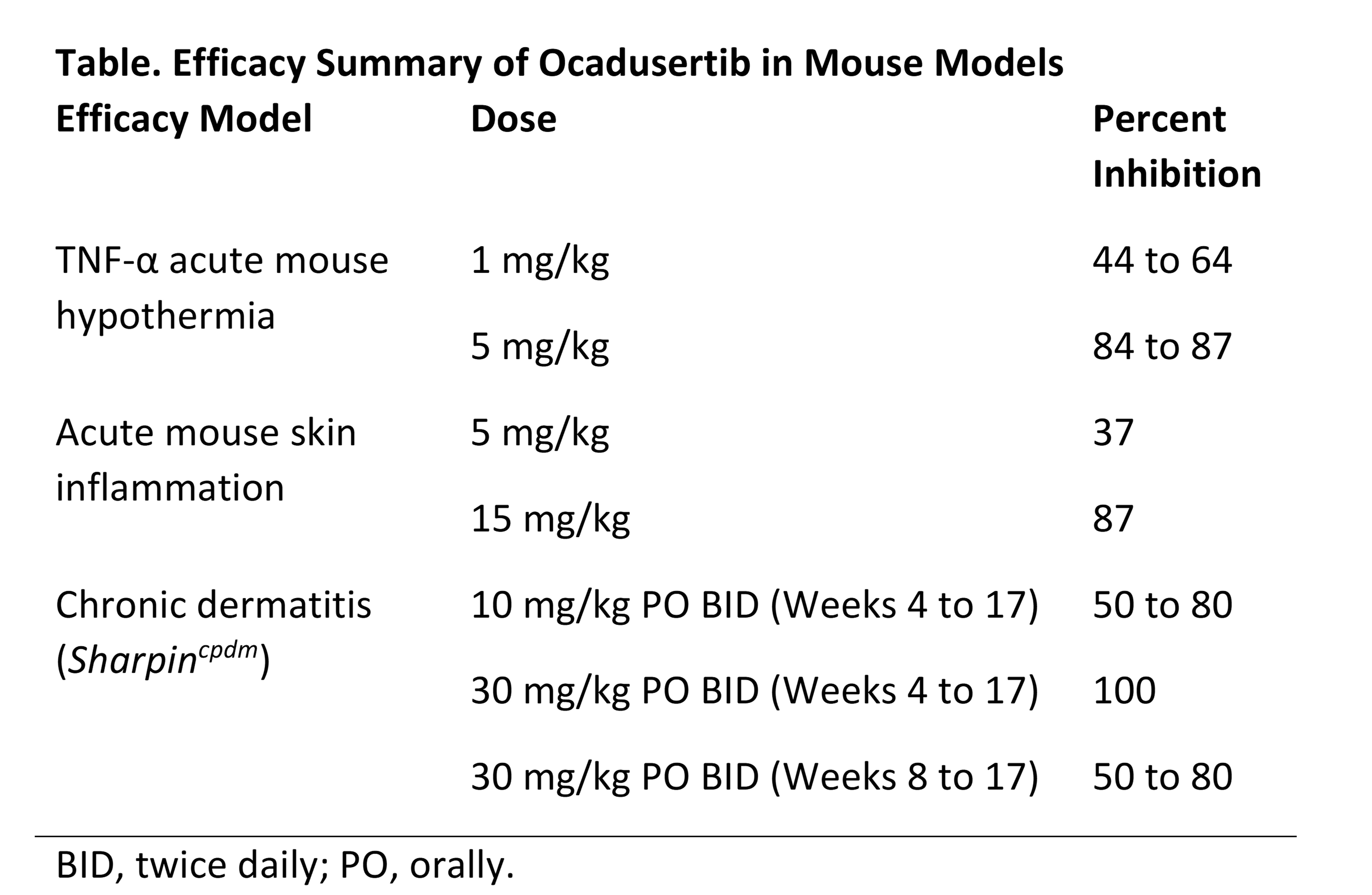
Results: Preclinically, the following results were observed: Ocadusertib inhibited RIPK1 enzymatic activity with a potency (half-maximal inhibitory concentration [IC50]) of 12 to 38 nM. Ocadusertib inhibited necroptotic responses in multiple in vitro immortalized and primary cell-based assays with a potency of 0.4 to 3 nM. Using a validated human whole blood assay, ocadusertib inhibited TNF/zVAD-induced cell death with a potency of 7 to 9 nM. Ocadusertib showed no significant inhibition in a panel of 105 kinases at 10 uM. In preclinical mouse efficacy studies, ocadusertib prevented RIPK1-dependent hypothermia in response to a TNF challenge; inhibited necroptosis-induced skin inflammation in an acute model; and was efficacious in a chronic proliferative dermatitis (Sharpincpdm) model, where treatment with ocadusertib resulted in a robust reduction in dermatitis severity (Table).
In a FIH Phase 1 study in healthy subjects, linear PK and dose-proportional exposure were observed with a time to maximum concentration of 1 to 4 hours and half-life (t1/2) of 13 to 15 hours (Figure). Steady state was attained at 4 to 6 days after multiple once-daily dose administrations.1
Ocadusertib was well tolerated in the FIH study, with no deaths, serious adverse events, treatment-emergent adverse events (TEAEs) leading to withdrawal, or other significant TEAEs reported. There were no clinically significant changes from baseline in laboratory assessments, electrocardiogram findings, or vital signs after ocadusertib treatment.
Conclusion: Preclinical studies have demonstrated ocadusertib to be a potent and selective allosteric inhibitor of RIPK1 inhibitor. In a Phase 1 study, ocadusertib had a long t1/2, was well tolerated, and had an acceptable safety profile. These findings support further assessment of ocadusertib in chronic inflammatory diseases including rheumatoid arthritis.
1. Yan L, et al. Abstract PII-042. Clin Pharmacol Ther. 2021;109(Suppl 1):S5-S88.
To cite this abstract in AMA style:
Hanson E, Dairaghi D, Vendel A, Sims J, Takita Y, Yan L, Chow A, Shaw S, Masuda e. Early Development of Ocadusertib, a Selective Receptor-Interacting Serine/Threonine-Protein Kinase 1 Inhibitor [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/early-development-of-ocadusertib-a-selective-receptor-interacting-serine-threonine-protein-kinase-1-inhibitor/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-development-of-ocadusertib-a-selective-receptor-interacting-serine-threonine-protein-kinase-1-inhibitor/