Session Information
Date: Sunday, November 17, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In the 2022 ACR classification criteria for giant cell arteritis (GCA), temporal artery ultrasound (TAU) is granted the same diagnostic value as temporal artery biopsy (TAB). And there is increasing the importance of non-invasive imaging studies in the diagnosis of GCA. However, there are limited reports on the role of those in Japanese patients.
We created a multicenter cohort of patients with GCA who were treated with tocilizumab (TCZ) to evaluate the effectiveness and safety of TCZ over a 2-year period. This secondary analysis aimed to examine the performance of imaging tests, especially TAU, in GCA diagnosis.
Methods: We conducted a multicenter, retrospective study. Patients with GCA who started TCZ at seven hospitals in Japan from January 2008 to July 2021 were extracted. We included the patients who were clinically diagnosed GCA according to the 1990 ACR criteria by rheumatologists at each institution.
We gathered information about patient demographics, GCA symptoms, and diagnostic methods at the time of diagnosis. TAU was evaluated by rheumatologists at each institution. CT, MRI, and PET-CT findings were evaluated by rheumatologists and radiologists at each institution. Pathological findings were evaluated by rheumatologists and pathologists at each institution.
Results: We identified 62 patients with GCA, with a median age of 74 years, of whom 65% were female. 75.8% (47/62) GCA met 1990 ACR classification criteria for GCA. Common symptoms at GCA diagnosis included headache (73%), polymyalgia rheumatica (62%), fever (32%), jaw claudication (26%) and scalp tenderness (23%).
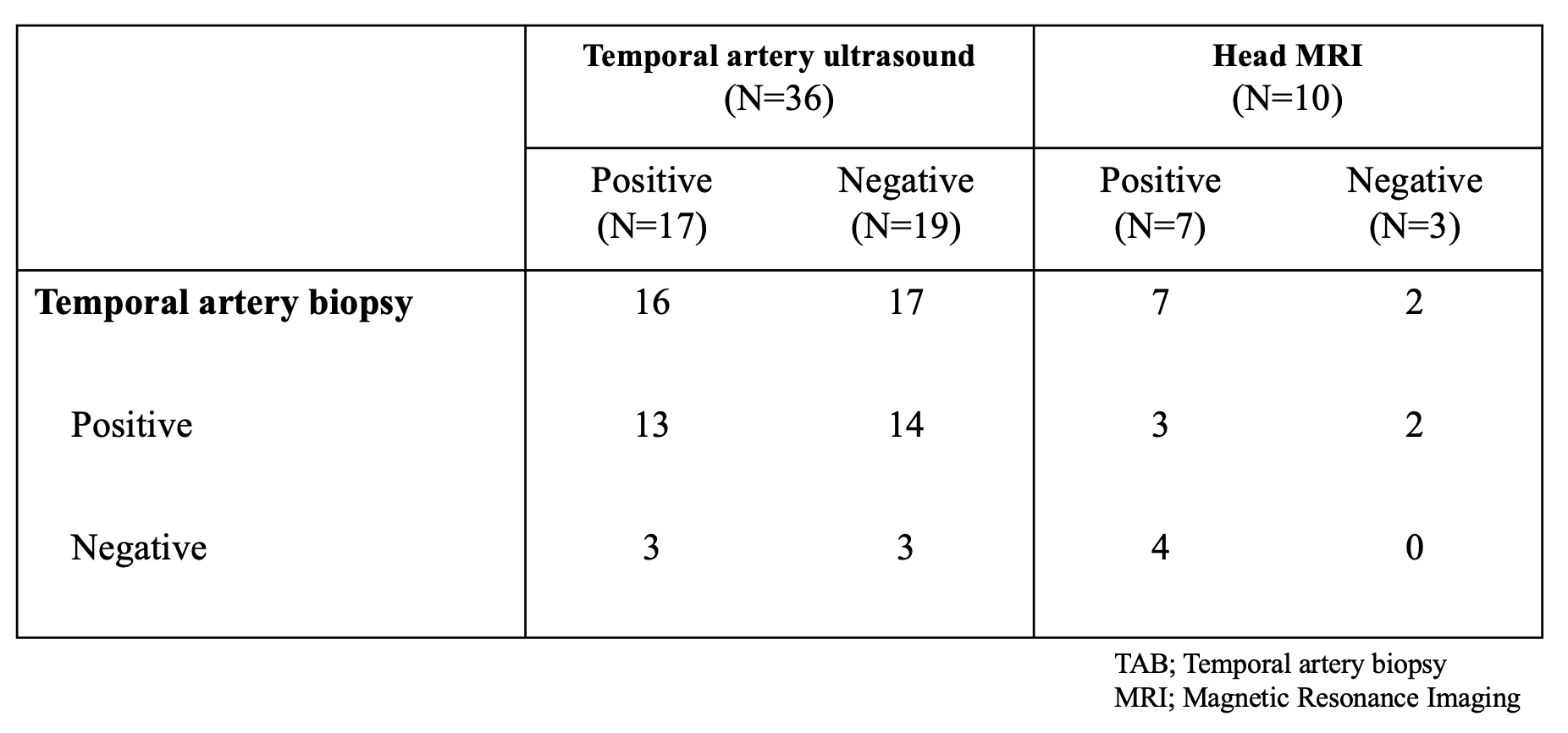
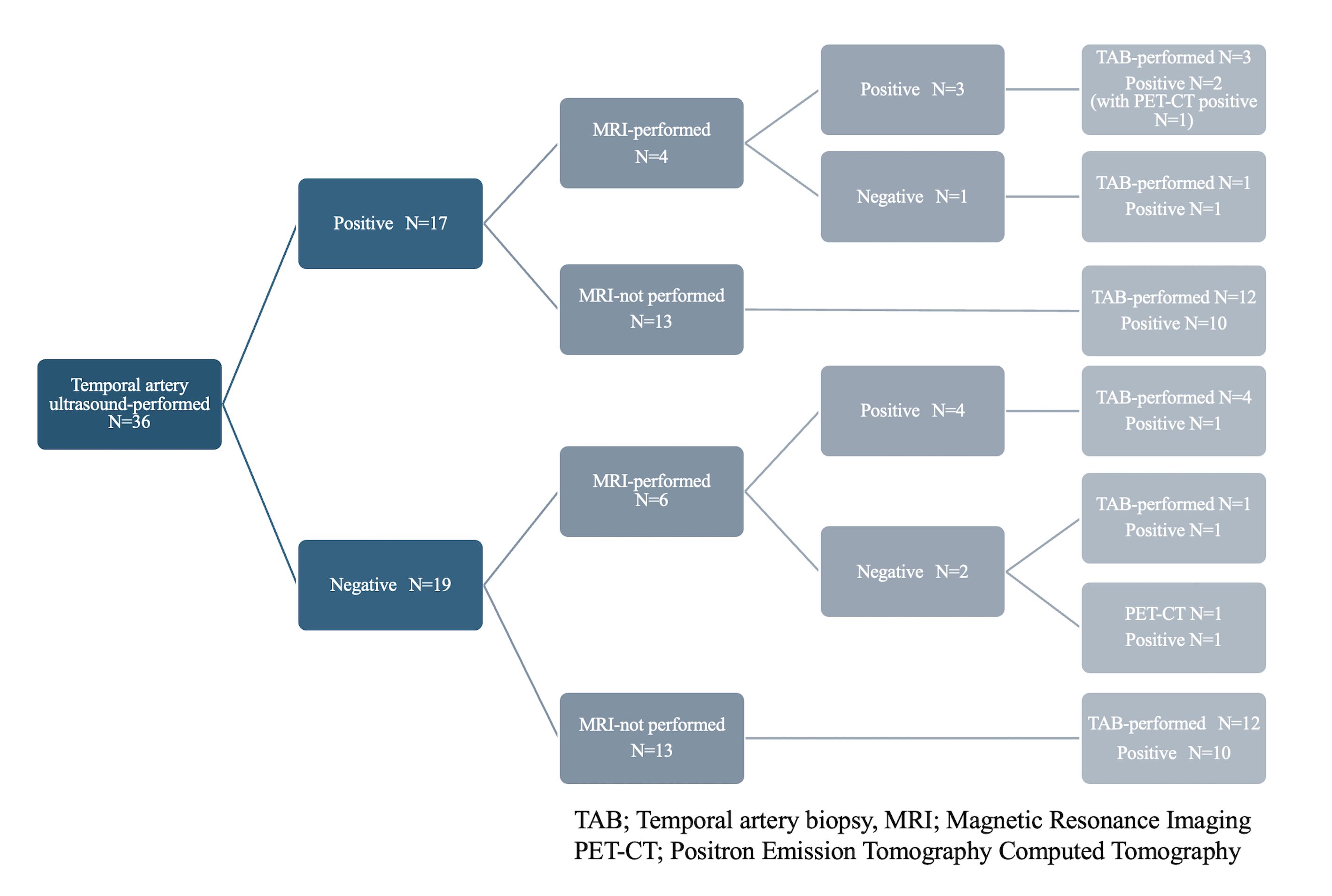
The positive rates for findings suggestive of GCA were 41% (22/54) for CT, 94% (16/17) for PET-CT, 47% (17/36) for TAU, 70% (7/10) for head MRI and 80% (35/44) for TAB. Among patients with negative ultrasound results, 17 underwent TAB, and 82.4% (14/17) were positive. Among patients with negative head MRI results, 2 underwent TAB, and 100% (2/2) were positive (Table). A detailed examination of patients who underwent TAU showed that head MRI was performed in 31.6% (6/19) of those with negative TAU, with 33.3% (4/6) testing positive (Figure). In the MRI-negative group, one patient underwent TAB and tested positive. In the group of MRI-not performed, 92.3% (12/13) underwent TAB, and 83.3% (10/12) were positive. PET-CT was performed in 2 cases. One of these cases involved an MRI-negative patient who did not underwent TAB, and the result was positive.
Conclusion: This multicenter observational study demonstrated the positive rates of imaging tests in real-world clinical setting. Focusing on TAU, although the test was negative in about half of the patients, most of the patients with negative ultrasound results had positive findings in other imaging tests. This implicates an important role of comprehensive imaging evaluation in diagnosing GCA
To cite this abstract in AMA style:
Oda N, Fukui S, Yamaguchi T, Nagase F, Ito T, Watanabe M, Haji Y, Suyama Y, Nomura A, Uechi E, Suda M, Takizawa N, Rokutanda R, Tamaki H. Real-World Data on Imaging Tests, Specifically Temporal Artery Ultrasound, in Giant Cell Arteritis in Japan: A Multicenter Retrospective Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-data-on-imaging-tests-specifically-temporal-artery-ultrasound-in-giant-cell-arteritis-in-japan-a-multicenter-retrospective-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-data-on-imaging-tests-specifically-temporal-artery-ultrasound-in-giant-cell-arteritis-in-japan-a-multicenter-retrospective-study/