Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-RNA polymerase III antibody (ARA) is associated with systemic sclerosis (SSc). The presence of ARA in patients with SSc is associated with the risk of complications such as diffuse skin involvement, scleroderma renal crisis (SRC), tendon friction rubs, gastric antral vascular ectasia (GAVE), and malignancy. Understanding the clinical significance of weak positive ARA is important to guide patient care regarding prognosis and monitoring. ARA can also be found in other systemic autoimmune (AI) diseases such as systemic lupus erythematosus (SLE), Sjogren disease (SjD), and rheumatoid arthritis (RA). This study aims to assess the relationship between weak positive ARA and the development and complications of SSc as well as the risk of other AI diseases.
Methods: A retrospective chart review was conducted at a single tertiary center. All patients between 18-80 years who had weak positive ARA (20-40 U) from 1/1/2013 to 12/31/2020 were included in the study. The ARA test utilized an enzyme immunoassay technique measuring ARA against RNA-polymerase III RP155 epitope. In patients who had multiple ARA tests at the time of follow-up, only those whose highest value was in the weak positive range were included. The time of follow-up continued until 2/8/2024. Information was abstracted including demographics, additional serology, rheumatologic diagnoses as made by a rheumatologist in the process of clinical care, complications of systemic sclerosis including pulmonary hypertension (PH), interstitial lung disease (ILD), tendon friction rubs, GAVE, and malignancy with their timing relative to ARA. Descriptive statistics were utilized to summarize data.
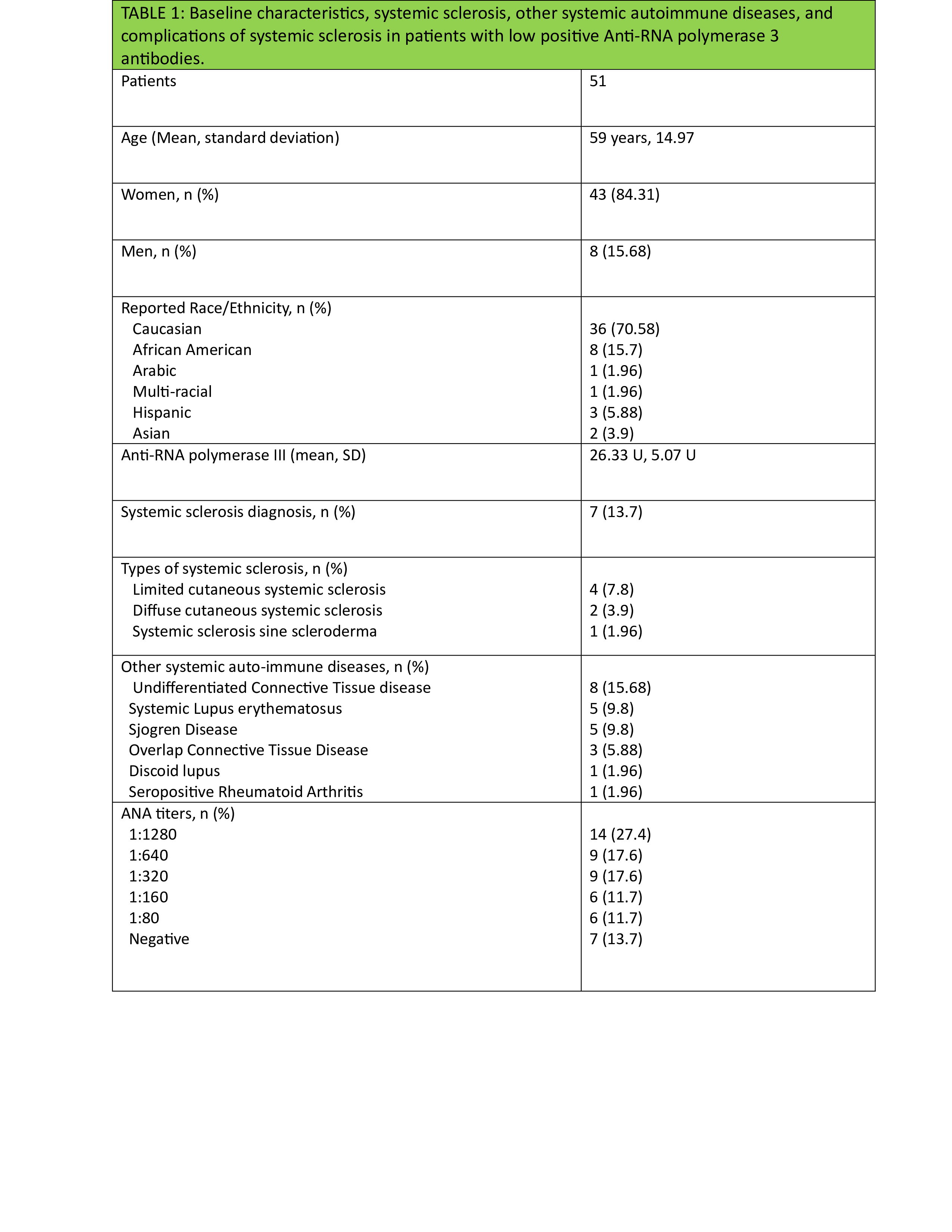
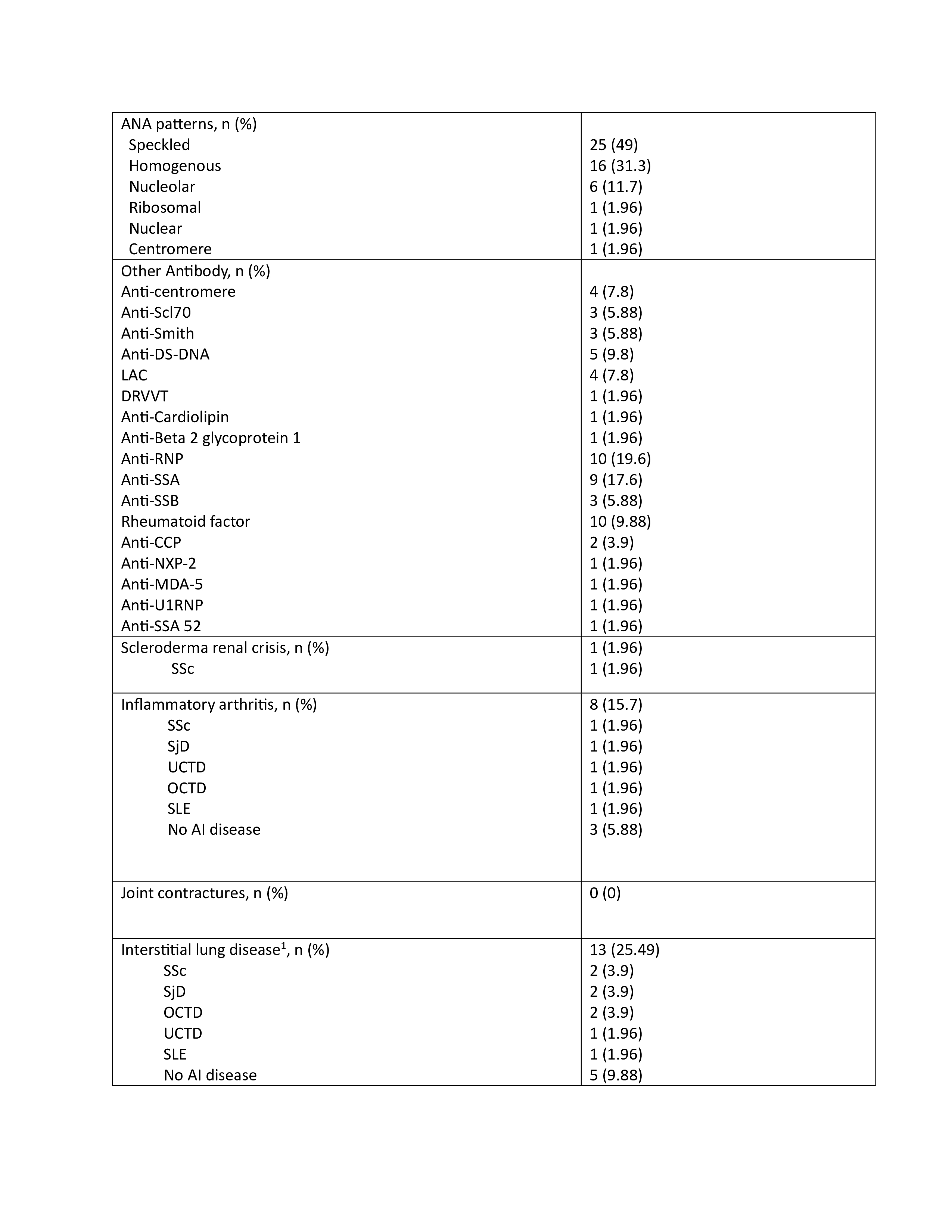
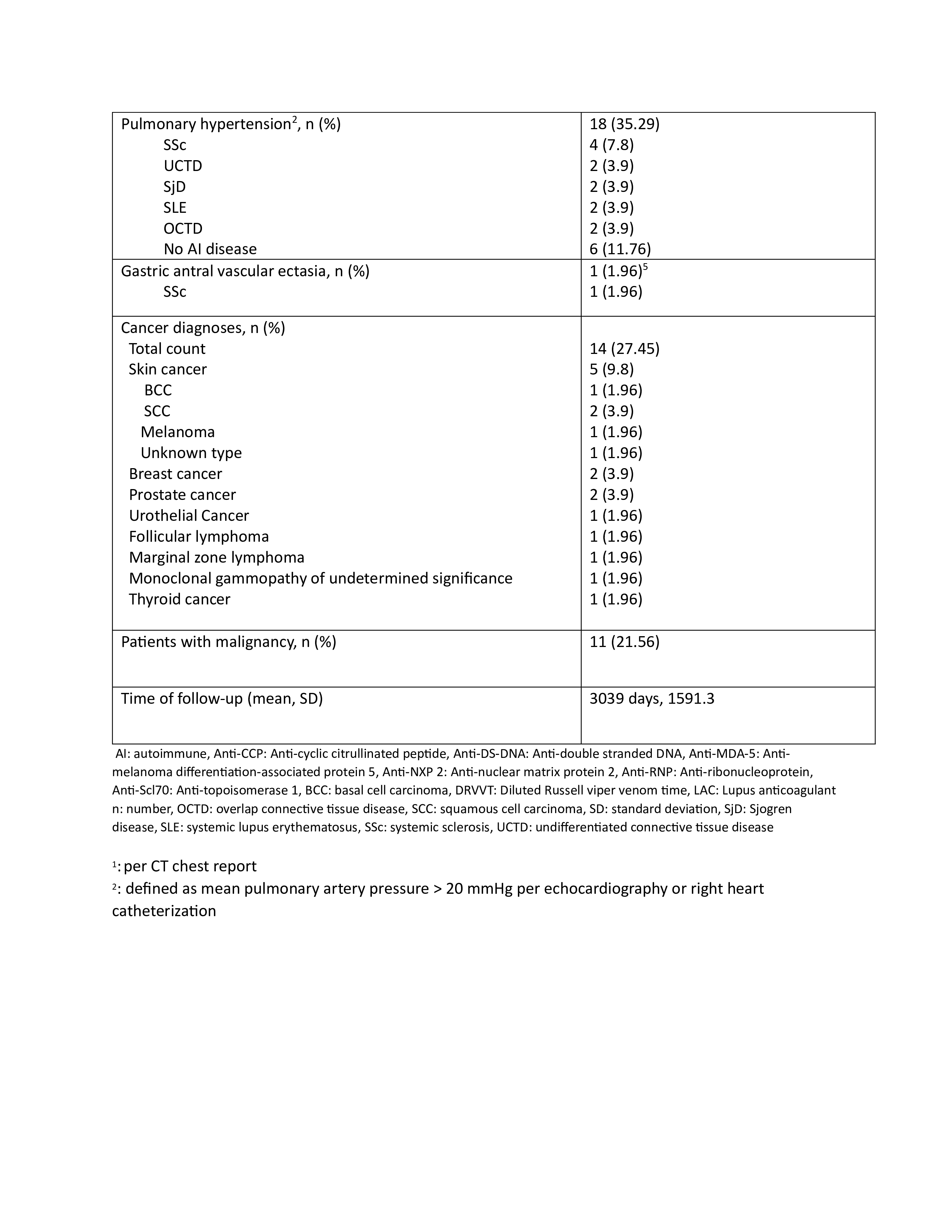
Results: Fifty-one patients, with 43 women (84%), had a weak positive ARA at the time of the study. The mean age was 59 at the time of ARA serology. Among 51 patients, 7 (14%) developed SSc over the time of follow-up (limited cutaneous SSc 4, diffuse cutaneous SSc 2, and systemic sclerosis sine scleroderma 1). Among the studied population, SRC and GAVE developed in 1 patient each (2%), while inflammatory arthritis developed in 8 (16%), interstitial lung disease (ILD) in 13 (25%), and pulmonary hypertension(PH) in 18 (35%) patients. Malignancy was noted in 11 (21.56%) patients, with 3 (5.88%) occurring after positive ARA. Over the course of follow-up, 8 (16%) patients developed undifferentiated connective tissue disease, 5 (10%) SLE, 5 (10%) SjD, and 3 (6%) overlap connective tissue disease. The most common anti-nuclear antibody (ANA) pattern was speckled, developing in 25 (49%) patients. Other autoantibodies found more frequently with weak positive ARA included rheumatoid factor in 10 patients (20%), anti-ribonucleoprotein antibody in 10 (20%), and anti-SSA in 9 (18%). The average time of follow-up was 8.32 years.
Conclusion: While this study indicates a relatively low rate of development of SSc in the setting of weak positive ARA, there was significant development of end-organ dysfunction including PH, ILD, and inflammatory arthritis in addition to other connective tissue diseases apart from SSc. This has important implications in terms of monitoring for patients with weak positive ARA even in those with a diagnosis of SSc is not made at the time of the antibody testing.
To cite this abstract in AMA style:
Badshah M, Balaja W, Donovan B, Schmidt P, Krause M. The Association of Weakpositive Anti-RNA Polymerase III Antibodies with the Diagnosis and Complications of Systemic Sclerosis and Other Systemic Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-association-of-weakpositive-anti-rna-polymerase-iii-antibodies-with-the-diagnosis-and-complications-of-systemic-sclerosis-and-other-systemic-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-association-of-weakpositive-anti-rna-polymerase-iii-antibodies-with-the-diagnosis-and-complications-of-systemic-sclerosis-and-other-systemic-autoimmune-diseases/