Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: B-cell depletion has been used to treat patients with systemic lupus erythematosus (SLE) for over twenty years, but we still lack studies that evaluate damage progression. Earlier acquisition of damage both within a year and a decade of the diagnosis of SLE are indicators of increased risk of mortality. In this study we explored the effectiveness of rituximab in restricting damage acquisition.
Methods: From an initial cohort of 850 SLE patients under long term follow up for the last 45 years, 380 were selected – 190 patients treated with rituximab and 190 with standard therapies – to compare their SLICC/ACR damage index (DI) results. We conducted a secondary analysis with 111 patients to evaluate DI progression during follow up – one rituximab patient matched with two counterparts based on sex, age at onset, type of SLE and ethnicity. This study was an audit and an observational retrospective study, not requiring formal hospital ethics approval or informed consent. Data was gathered using relative and absolute proportions; for group comparisons, Pearson’s chi-square test or Fisher’s exact test, as well as Student’s t-test for parametric data or Mann-Whitney test for nonparametric data were used. For the secondary analysis we used a generalized estimating equation. A p-value of less than 0.05 was considered
statistically significant. Analyses were conducted using SPSS software.
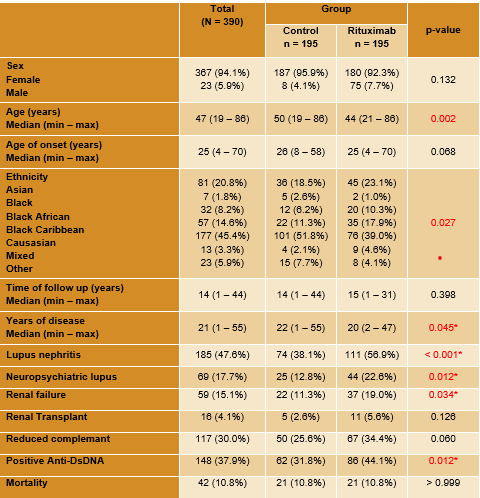
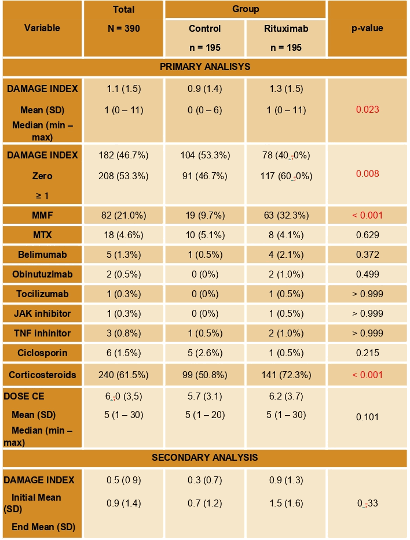
Results: The majority of patients were female (94.1%), caucasian (45,4%) and with a median age of 47 years. Severe disease manifestations and higher titers of anti-dsDNA antibodies (86 U/ml vs 62 U/ml; p=0.012) were seen in the rituximab group (Table 1). Use of mycophenolate mofetil (32.3% vs. 9.7%; p< 0,001) were higher in the rituximab group. No difference was seen in other immunosuppressive or biologic drugs. Corticosteroids use at the end of the follow up was higher in the rituximab group (72.3% vs 50.8%; p< 0,001), but steroid doses were similar (5.7mg vs 6.2mg; p = 0.101). The median SLICC/ACR DI was higher in the rituximab group (1.3 vs 0.9; p= 0,02). No difference between mortality was seen between groups (10.8% in both groups; p >0.99). In the secondary analysis the mean variation of SLICC/ACR DI at the beginning and at the end of the follow up was lower in the control group, with no statistical difference between the two groups (0.4 vs 0,6; p = 0.33). We have also identified a statistical significance
between the two groups regarding the proportion of patients that changed their DI during follow up (58.2% in the control group vs 44.2% in the rituximab) (Table 2).
Conclusion: As an effective B cell depleting therapy, rituximab is a valid therapeutic option for SLE patients, especially in those with refractory or life-threatening manifestations, such as lupus nephritis and neuropsychiatric lupus, as shown in our cohort. Patients treated with rituximab had more severe disease and therefore a higher SLICC/ACR DI from the start of the follow up. However, there was no difference between progression of median SLICC/ACR DI between groups, as there was a lower progression of damage in patients treated with rituximab. Rituximab was thus shown to slow the rate of damage progression in patients with severe SLE.
To cite this abstract in AMA style:
Brito A, Moitinho de Almeida T, Miranda S, Barreto Baié M, Calado D, Isenberg D. Effect of Rituximab on Long-term Damage Acquisition in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effect-of-rituximab-on-long-term-damage-acquisition-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-rituximab-on-long-term-damage-acquisition-in-patients-with-systemic-lupus-erythematosus/