Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Tacrolimus is a pregnancy-compatible immunosuppressive increasingly used in systemic lupus erythematosus (SLE) pregnancies. Physiological changes throughout pregnancy impact tacrolimus pharmacokinetics, altering the drug’s whole blood concentrations during gestation. However, data is very limited to guide clinicians caring for pregnant women receiving tacrolimus in interpreting tacrolimus trough levels and adjusting the dosage. We completed a systematic review focusing on the variations of maternal tacrolimus trough levels and dosage during SLE and non-SLE pregnancies.
Methods: Using a combination of relevant search terms and keywords, we systematically searched Embase, Ovid, PubMed, Web of Science and Cochrane Library up to January 2024. All observational studies which measured whole blood tacrolimus trough levels during pregnancy were included without language or date restriction. Studies which were reviews, case reports, abstracts only, randomized clinical trials, non-human, and had no tacrolimus levels during pregnancy were excluded from the review. We then used random-effects models to estimate standardized mean differences (SMD) or mean differences (MD), with 95% confidence intervals (CI) of tacrolimus trough levels and doses before and during pregnancy and in the postpartum.
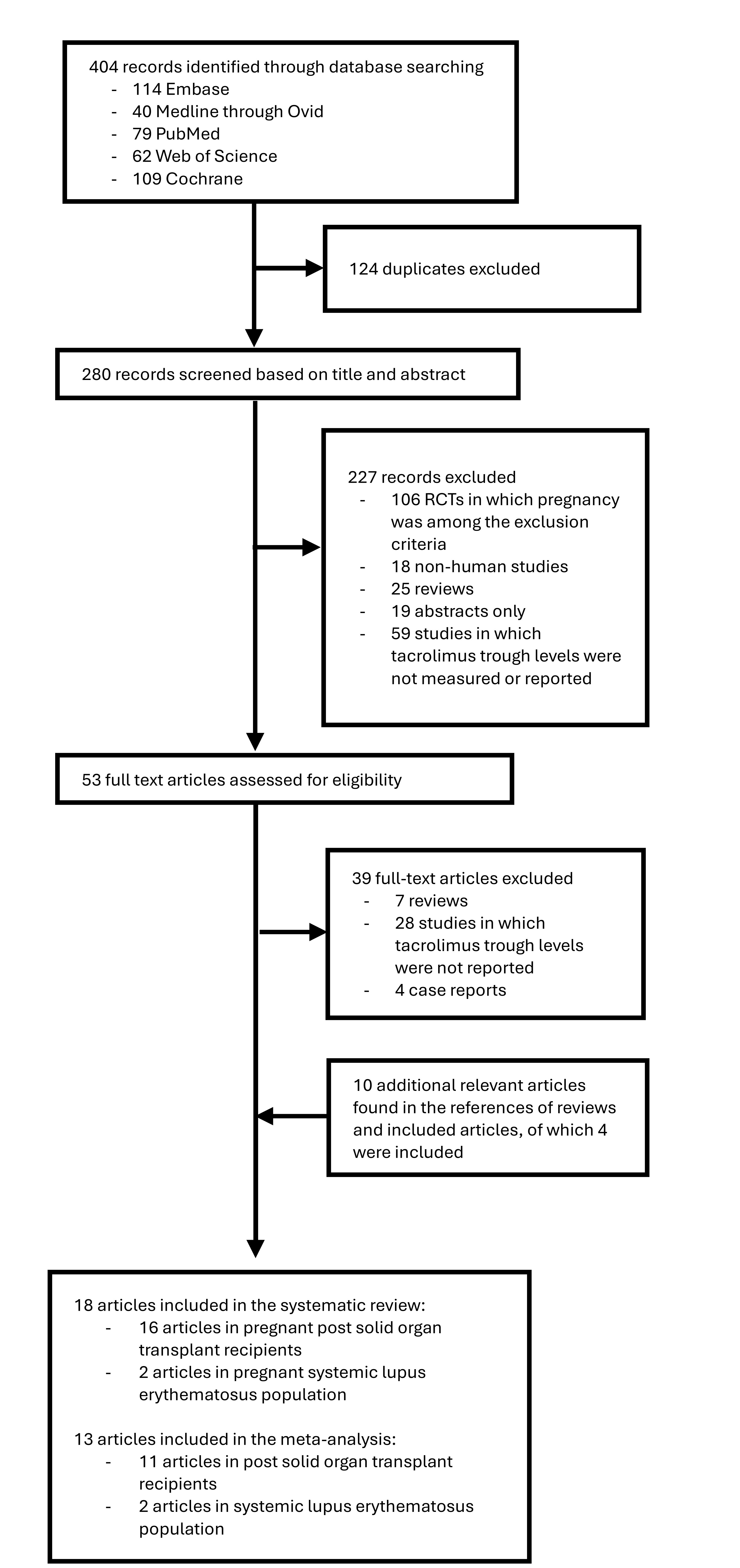
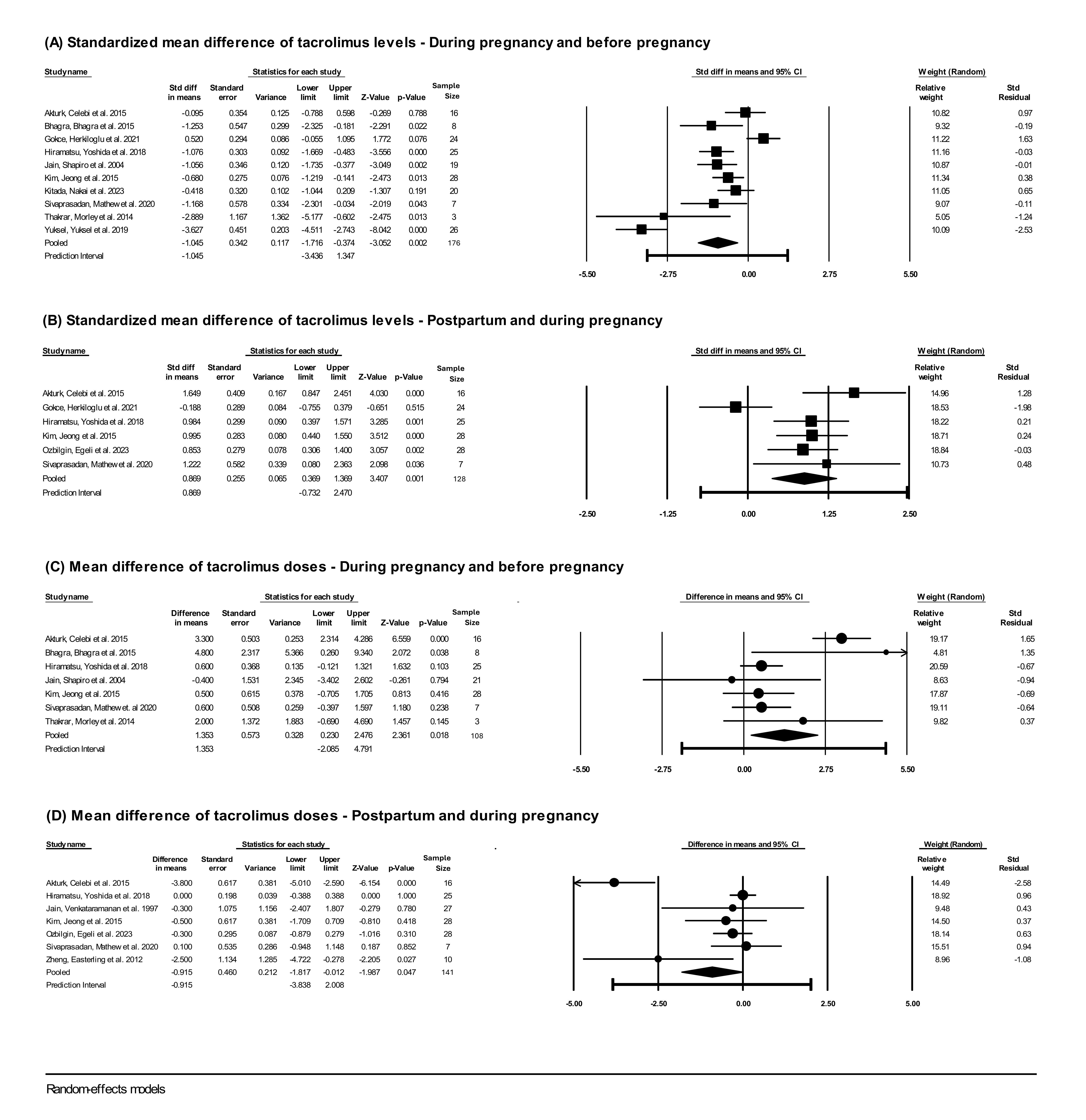
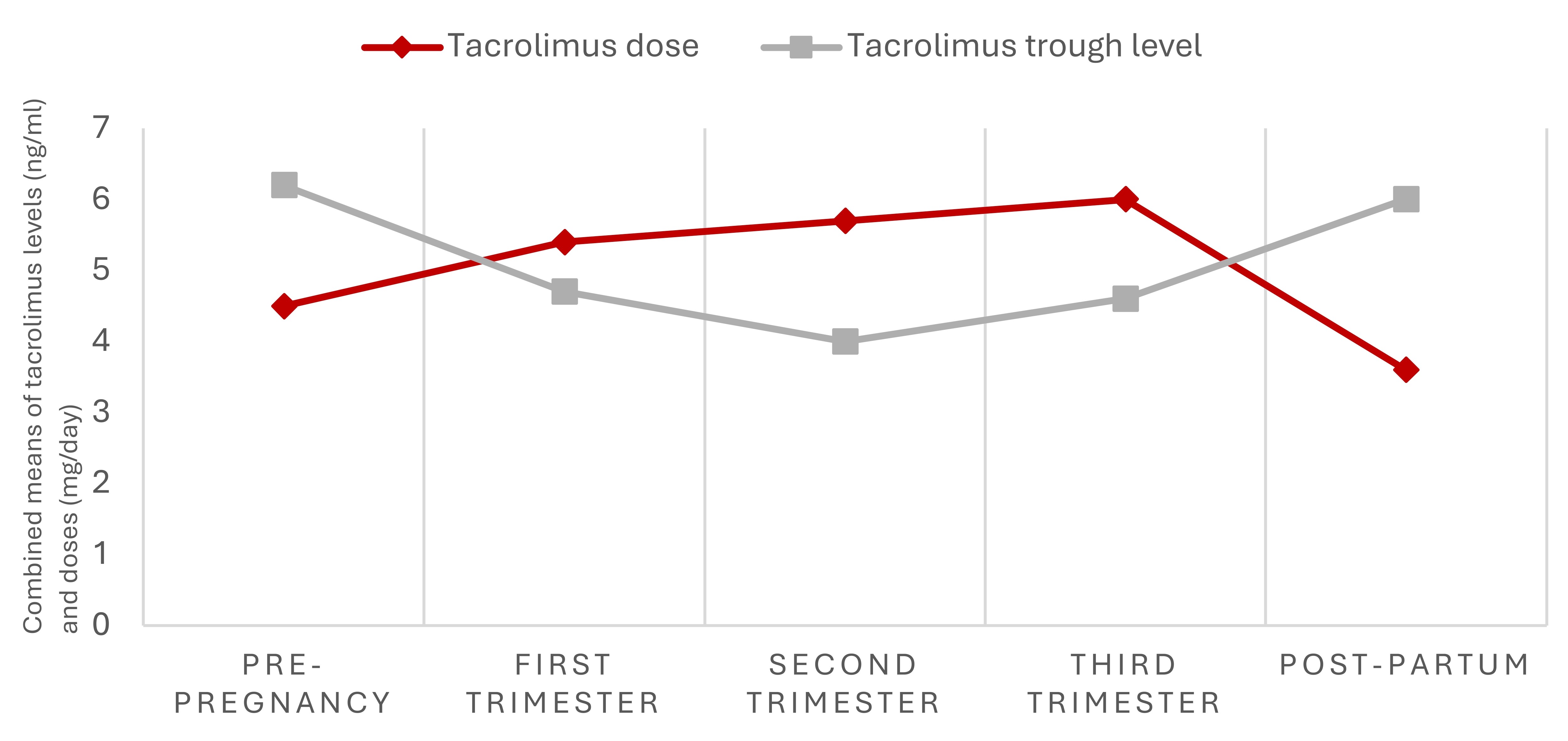
Results: Of 404 publications identified, 124 duplicates were excluded and 282 were screened based on title and abstract, of which 53 full-text articles were assessed for eligibility. Eighteen articles were included in the systematic review and 13 in the meta-analysis. Only 2 studies assessed tacrolimus levels in SLE pregnancies, while the remainder were in pregnant organ transplant recipients (Figure 1). Tacrolimus levels significantly decreased during pregnancy compared to pre-pregnancy (SMD -1.05; 95% CI -1.72, -0.37), and significantly increased in the postpartum compared to levels during gestation (SMD 0.87; 95% CI 0.37, 1.37) (Figure 2 A, B). Mean differences in tacrolimus trough levels were -1.56 ng/ml (95%CI -2.82, -0.31) between first trimester and before pregnancy, -0.49 ng/ml (95% CI -1.04, -0.07) between second and first trimesters, 0.63 ng/ml (95% CI 0.30, 0.96) between third and second trimesters, and 1.28 ng/ml (95% CI 0.60, 1.96) between the postpartum and third trimester. The variation in tacrolimus levels during pregnancy was usually addressed by increasing the dose during pregnancy vs pre-pregnancy (MD 1.35 mg/day, 95% CI 0.23, 2.48) and decreasing the dose in the postpartum vs pregnancy (MD -0.92 mg/day; 95% CI -1.8, -0.01) (Figure 2 C, D).
Conclusion: Tacrolimus whole-blood levels decrease in the first and second trimesters, then increase back to pre-pregnancy levels in the postpartum. Tacrolimus dosage in pregnancy is typically increased to maintain tacrolimus trough levels within usual therapeutic ranges. Increasing drug dosage could elevate the bio-effective fraction of tacrolimus (not measured by trough levels), raising concerns for safety and efficacy of dose augmentation during pregnancy. Further research is needed to guide clinicians in adjusting tacrolimus in SLE and non-SLE pregnancies to optimize therapeutic drug monitoring in high-risk populations.
To cite this abstract in AMA style:
Farhat R, Mendel A, Malhamé I, Grunbaum A, Bernatsky S, Vinet E. Variations in Tacrolimus Whole Blood Concentrations During Pregnancy and Its Implications for Therapeutic Drug Monitoring: A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/variations-in-tacrolimus-whole-blood-concentrations-during-pregnancy-and-its-implications-for-therapeutic-drug-monitoring-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/variations-in-tacrolimus-whole-blood-concentrations-during-pregnancy-and-its-implications-for-therapeutic-drug-monitoring-a-systematic-review-and-meta-analysis/