Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Nonsteroidal anti-inflammatory drugs (NSAIDs) are first line pharmacological treatment for Axial Spondyloarthritis (axSpA), yet individual responses to different NSAIDs vary significantly. This study aims to evaluate the efficacy and patient preference of different NSAIDs in axSpA management through a series of N-of-1 clinical trials to assess treatment effects.
Methods: This study conducted a series of N-of-1 trials involving 42 patients consecutive patients meeting ASAS criteria for AxSpA from a single, academic center. A series of N-of-1 trials is a set of individualized, crossover trials where each patient serves as their own control to identify the most effective treatment for their condition. Each patient underwent two cycles of randomized, double-blind, crossover comparisons of three NSAIDs: celecoxib 200 mg , meloxicam 7.5 mg , and naproxen 500 mg twice daily. The primary outcome was the change in the Ankylosing Spondylitis Disease Activity Score (ASDAS) from baseline. In the first cycle, each NSAID was administered for four weeks with outcomes assessed at the end of this 4-week medication. The least-preferred medication (based on adverse reactions to medication/least reduction in ASDAS) was then eliminated, and patients entered a second cycle, taking the two remaining NSAIDs again in a randomized order. The final, preferred NSAID drug was again chosen based on greatest mean reduction in ASDAS Bayesian analysis, assuming a neutral prior (no preference in the comparison), was used to calculate the mean change in ASDAS and posterior probabilities for each NSAID. Secondary outcomes included patient preference and adverse events.
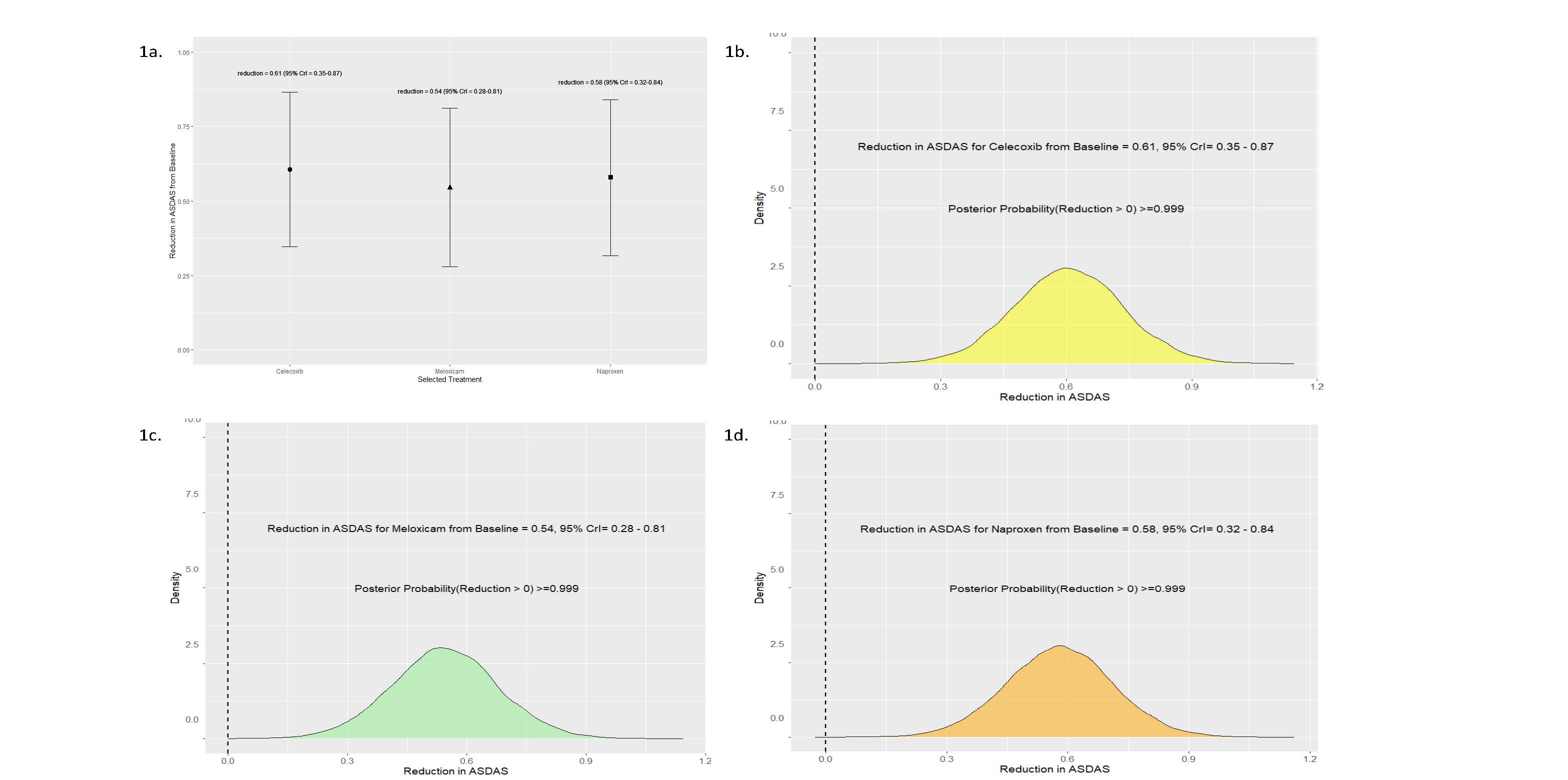
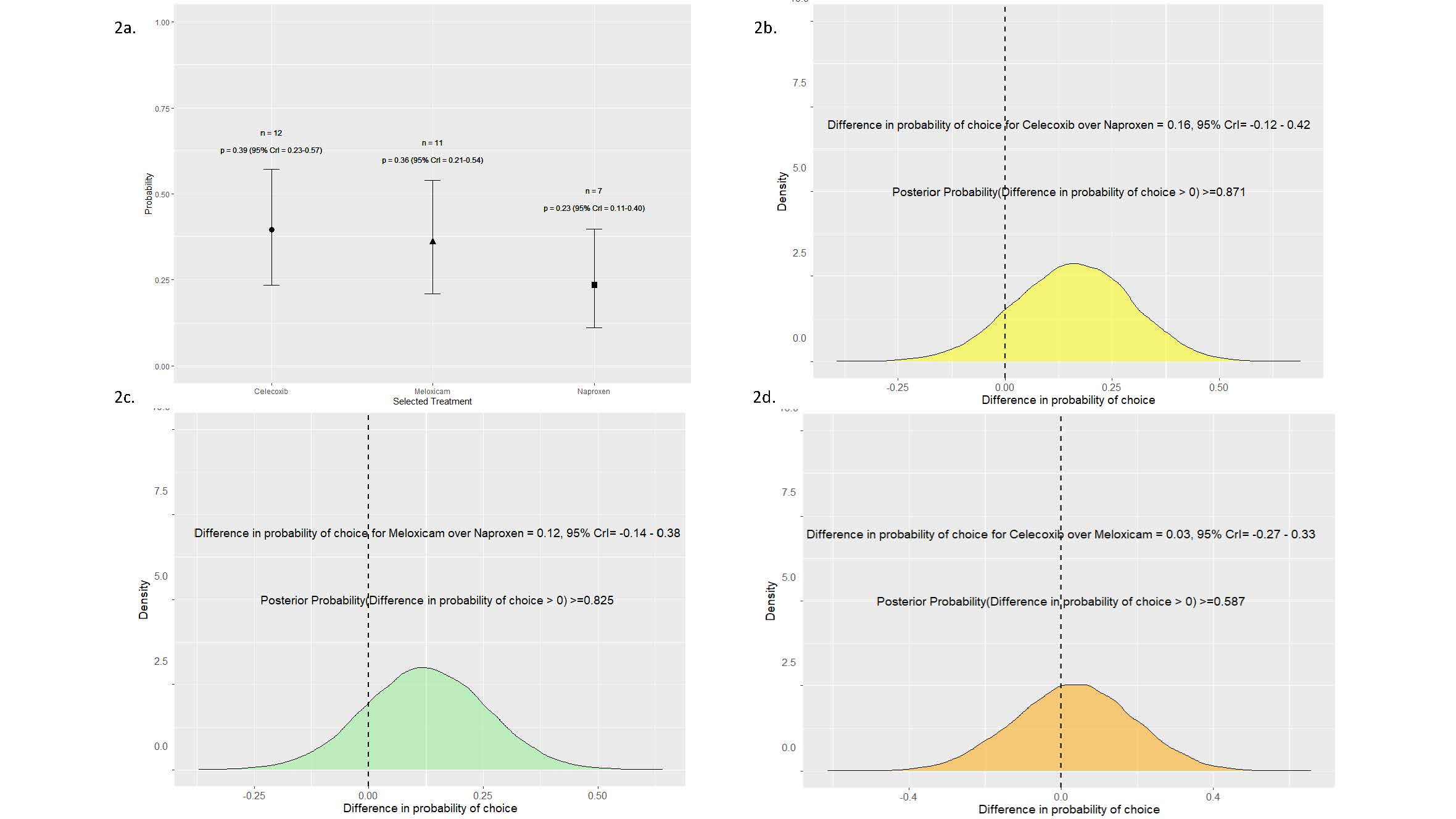
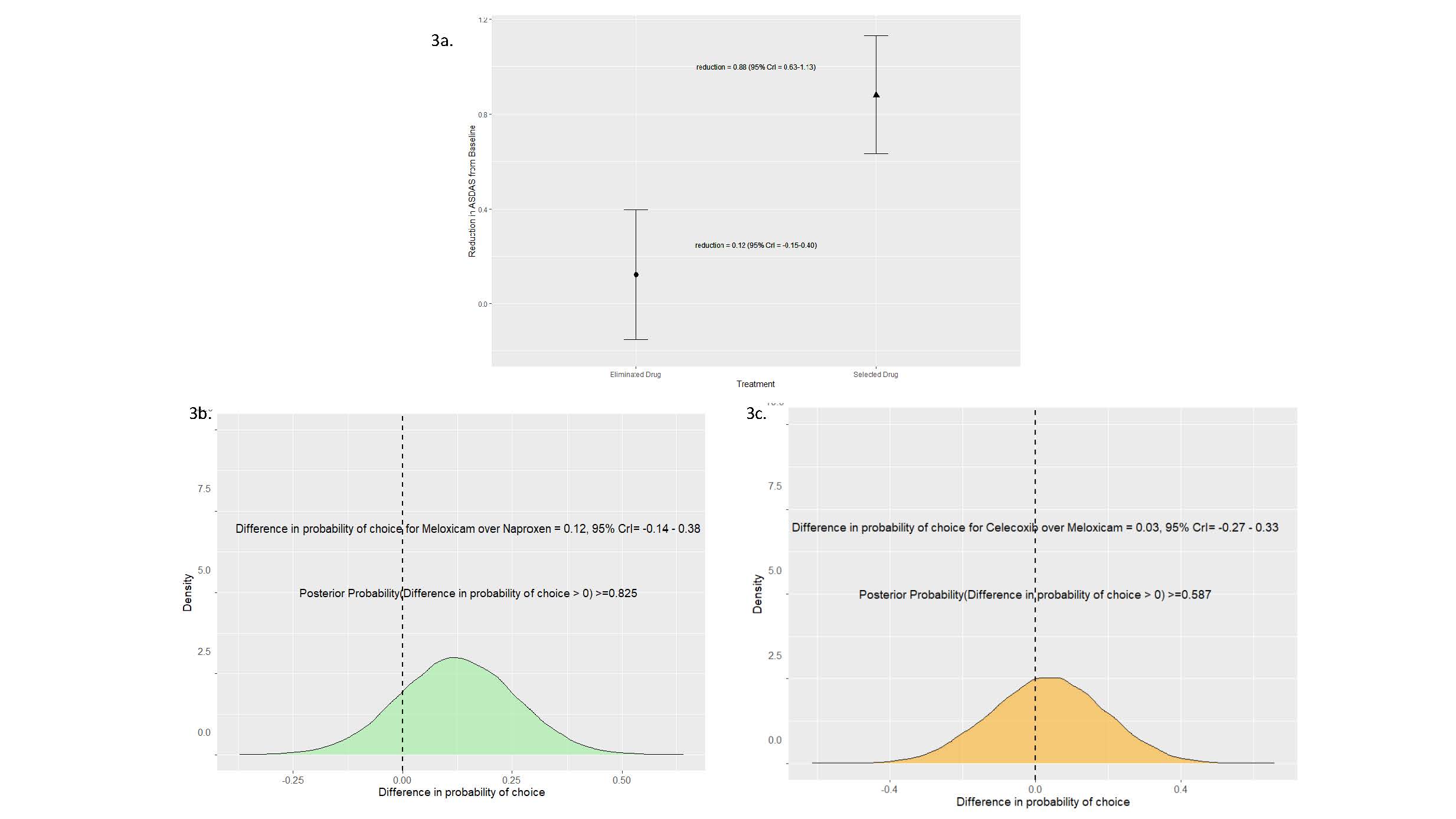
Results: Baseline demographics were gender parity (n=21 per gender) with a mean age of 43.14 years (± 14.26) and BMI of 30.79 (± 14.26). Thirty out of the 42 patients (72%) enrolled completed the N-of-1 trial series. Mean reductions in ASDAS from baseline conferred benefits for all NSAIDs (celecoxib, meloxicam, and naproxen) with high posterior probabilities of benefit ( >0.99) (Figure 1). No particular NSAID was chosen over the other NSAIDs as the preferred drug (Figure 2). Importantly, the preferred drug, identified based on individual patient responses, conferred a greater reduction in ASDAS compared to the eliminated drug (0.88 vs. 0.12, posterior probability of benefit >0.99) (Figure 3). Adverse events were infrequent and similar across all NSAIDs, with gastrointestinal issues being the most common.
Conclusion: Bayesian analysis in N-of-1 trials effectively identified the optimal NSAID for individual patients with axSpA, highlighting substantial variability in individual responses. Personalized NSAID therapy guided by this approach can improve clinical outcomes and patient satisfaction while minimizing adverse effects. This study supports the need for larger n-of-1 trials to examine substantial variability in individual responses to NSAIDs with the ultimate goal of broader implementation in clinical practice.
To cite this abstract in AMA style:
Hwang M, Kim S, Assassi S, Samuel J, Green C, Tyson J, Reveille J. Individualizing NSAIDs in Axial Spondyloarthritis Through a Series of N-of-1 Clinical Trials with Bayesian Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/individualizing-nsaids-in-axial-spondyloarthritis-through-a-series-of-n-of-1-clinical-trials-with-bayesian-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/individualizing-nsaids-in-axial-spondyloarthritis-through-a-series-of-n-of-1-clinical-trials-with-bayesian-analysis/