Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Elevated baseline (BL) C-reactive protein (CRP) level can serve as a predictor for treatment response to TNF inhibitors in patients (pts) with r-axSpA. The impact of CRP on b/tsDMARD response in r-axSpA is listed as a topic of interest on the ASAS-EULAR research agenda1. Ixekizumab (IXE) has previously demonstrated efficacy in the treatment of r-axSpA in pts with normal and elevated CRP 2. This analysis further investigates the impact of baseline CRP levels on the ASAS40 individual components, BASDAI, and Axial Spondyloarthritis Disease Activity Score (ASDAS).
Methods: Biologic naive adults with r-axSpA were enrolled in the COAST-V study (NCT02696785). At BL, pts were randomised to treatment with IXE Q4W, adalimumab (ADA), or placebo (PBO). Randomisation was stratified by baseline (BL) CRP normal (CRP ≤5 mg/L) or elevated (CRP >5 mg/L). This analysis reports change from BL (CFB) at 16 weeks for ASAS40 individual components, BASDAI, and ASDAS responses. Descriptive results for these outcomes are presented according to CRP levels.
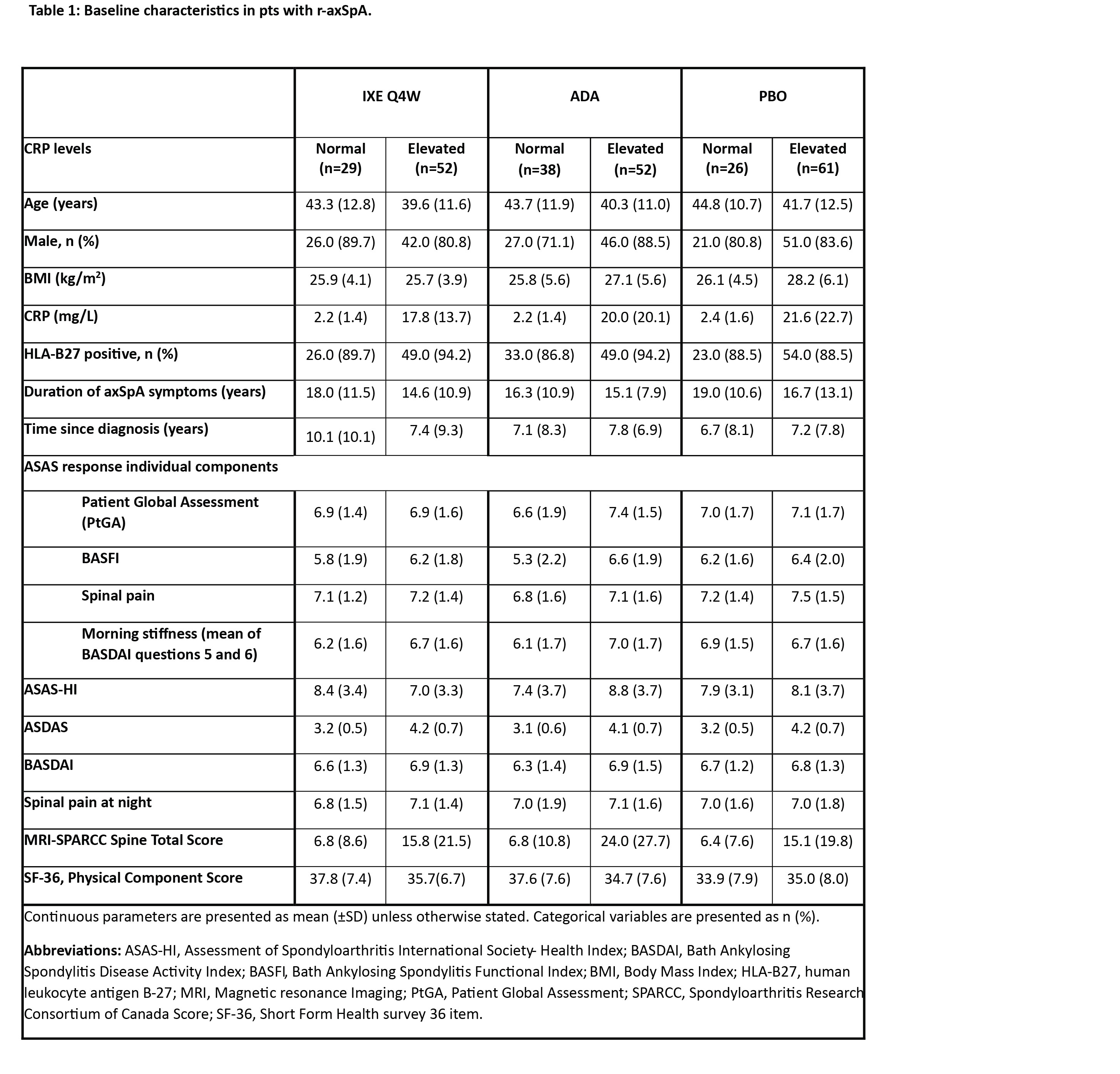
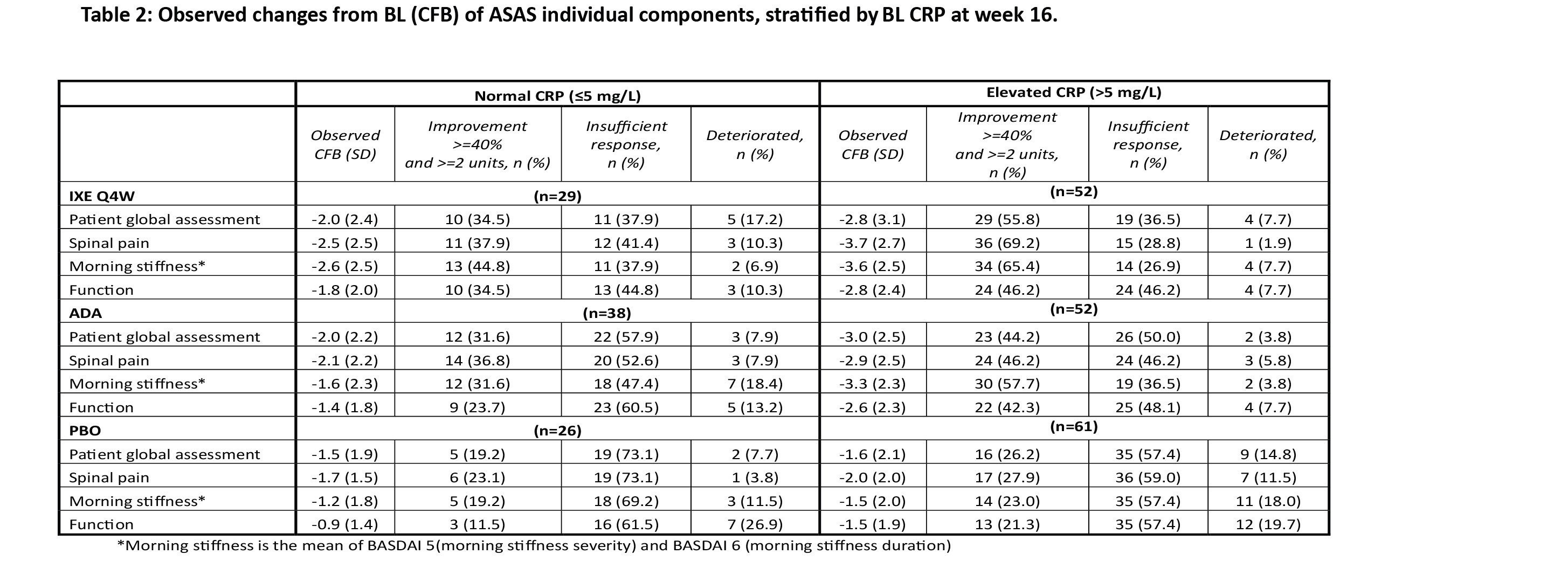
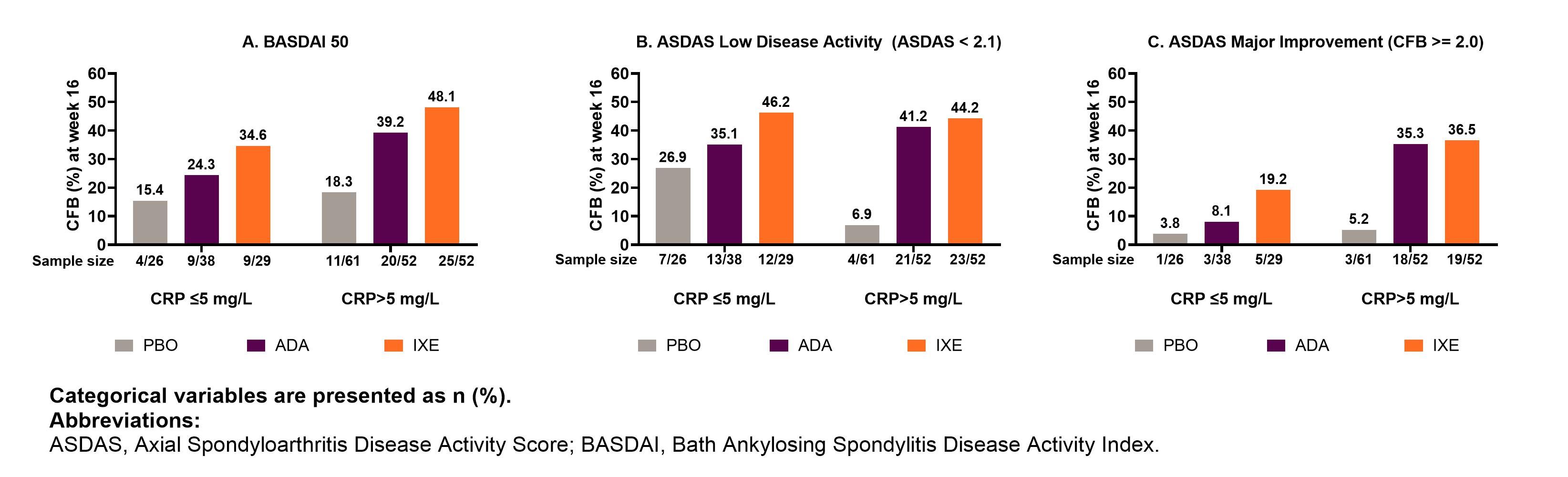
Results: The mean ages of pts with normal CRP at BL were 43.3, 43.7, and 44.8 years for IXE, ADA, and PBO respectively (subsequently reported in that order, herein), whereas the mean age of pts with elevated CRP at BL was 39.6, 40.3, and 41.7 years. Gender, HLA-B27 positivity distribution and the mean duration of axSpA diagnosis was similar across both CRP groups (Table 1). Pts with normal CRP had lower mean MRI-SPARCC spine score vs those with elevated CRP (6.8, 6.6, and 6.4 vs 15.8, 24.0, and 15.1 units) for IXE, ADA, and PBO respectively. At BL disease activity was comparable between pts with normal and elevated CRP. In pts treated with IXE, ASAS40 response at week 16 was driven by all 4 individual components with the largest improvements seen in morning stiffness (mean of intensity and duration of morning stiffness questions of BASDAI) and spinal pain, across both normal and elevated CRP (Table 2). BASDAI 50 was achieved by 34.6%, 24.3%, and 15.4% of pts with normal CRP and by 48.1%, 39.2%, and 18.3% of pts with elevated CRP. ASDAS major improvement was achieved by 19.2% (IXE), 8.1% (ADA) and 3.8% (PBO) of pts with normal CRP and by 36.5%, 35,3% and 5.2% of pts with elevated CRP, respectively (Figure 1C).
Conclusion: In pts with r-axSpA, BL clinical characteristics were similar in the normal and elevated CRP groups with pts experiencing similar disease burden. Across IXE and ADA treatment groups, the elevated CRP group tended to achieve higher treatment responses than the normal CRP group. Whereas in patients with normal CRP, CFB of ASAS individual components, BASDAI and ASDAS improvement was numerically highest in IXE Q4W followed by ADA and PBO.
References:
< !1. Ramiro et al. Ann Rheum Dis. 2023; 82(1):19-34.
< !2. Maksymowych et al. Rheum. 2022; (11):4324-4334.
To cite this abstract in AMA style:
Sengupta R, Machado P, Goupille P, Navarro Compán V, Xu H, sheesh m, Jing Ng K, Ngantcha M, Russ H, Doridot G, Baraliakos X. Baseline Characteristics and Efficacy in Patients with Radiographic AxSpA (r-axSpA) Stratified by CRP Level: An Analysis from the Ixekizumab Phase III Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/baseline-characteristics-and-efficacy-in-patients-with-radiographic-axspa-r-axspa-stratified-by-crp-level-an-analysis-from-the-ixekizumab-phase-iii-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-and-efficacy-in-patients-with-radiographic-axspa-r-axspa-stratified-by-crp-level-an-analysis-from-the-ixekizumab-phase-iii-trial/