Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: XKH004, the first recombinant anti-human IL-17A/F humanized monoclonal antibody in China (also known as XKH004), shares the same therapeutic target as Bimekizumab from UCB pharma, the first biologic targeting IL-17 A/F (XKH004 is the second one worldwide). In the Phase 1 clinical trial, XKH004 demonstrated favorable safety and efficacy profiles in healthy volunteers. In this article, we present the results of a Phase 2 study of XKH004 (CTR20212135).
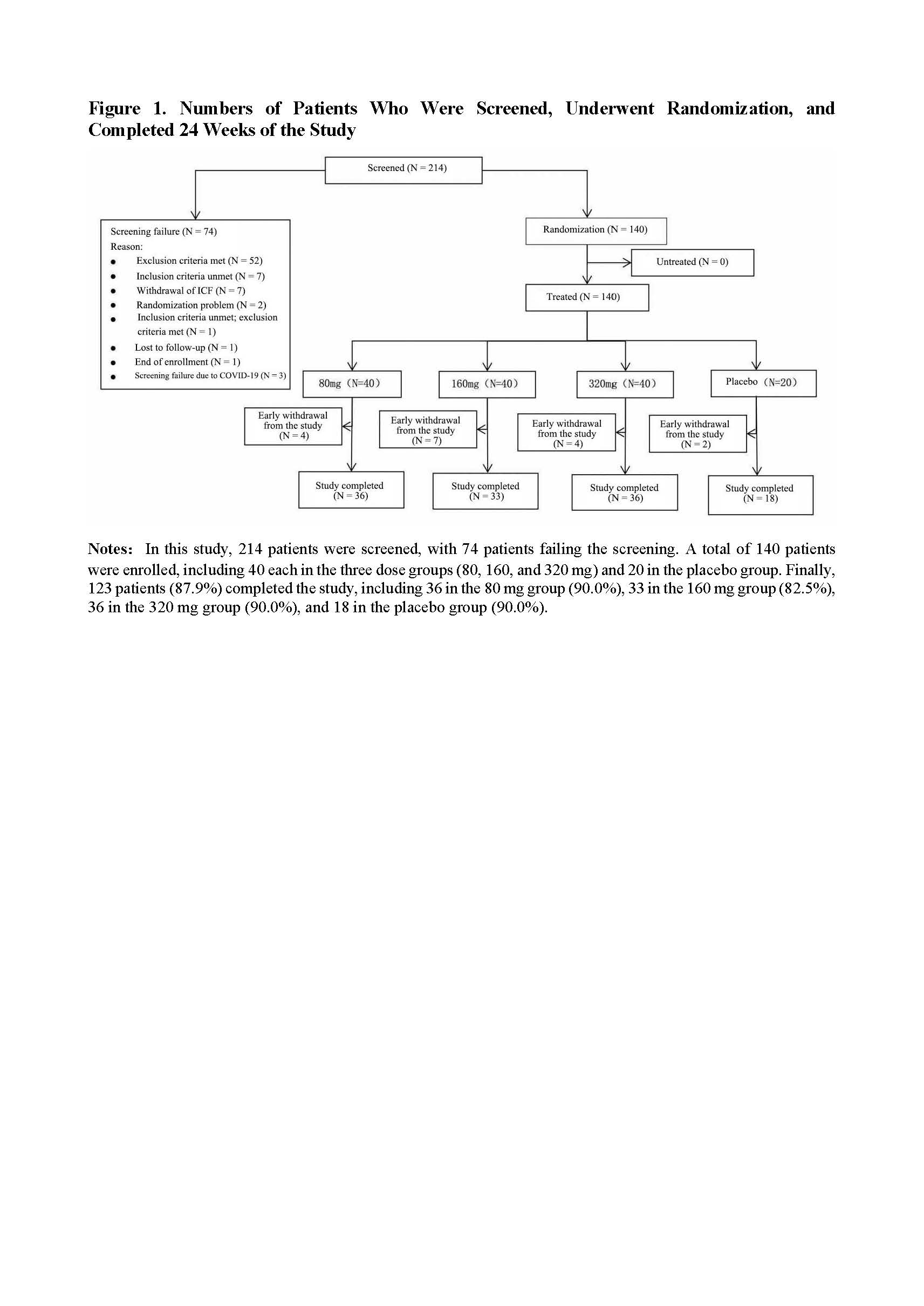
Methods: The AS patients included in the study met the 1984 New York criteria, were aged between 18 and 60 years, and had ≥4 scores of Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and total back pain. Totally 140 patients were randomized in a 2:2:2:1 ratio to receive XKH004 at doses of 80, 160, or 320mg (with a loading dose of 320mg, followed by 160mg doses) or placebo. The primary endpoint was the Assessment of SpondyloArthritis International Society ≥40% improvement (ASAS40) response rate at week 16, while secondary endpoints included ASAS20 response rate, BASFI, BASDAI, BASMI, MASES, ΔCRP, ΔESR, total back pain, morning stiffness score, safety, and immunogenicity.
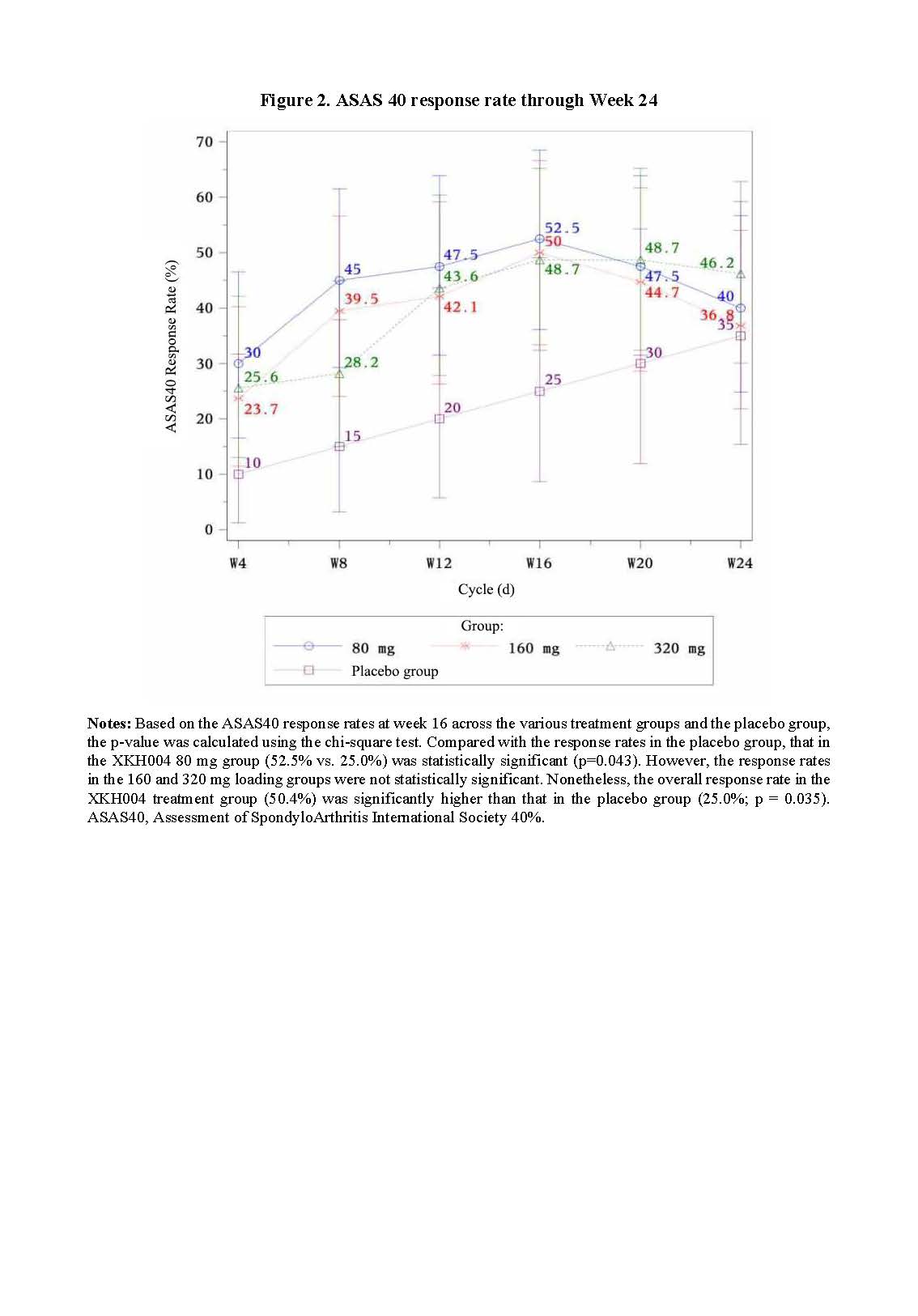
Results: At week 16, 50.4% of all patients in XHK004 group achieved ASAS40 at W16, showing a significant difference compared to the placebo group (25%) (P = 0.035). However, only the response rate of 80mg group (52.5%) was significantly higher than the placebo group (25.0%) (P=0.043).For endpoint of ASAS20, 70.0% of patients in the XKH004 80 mg group and 69.2% in the 320 mg group achieved it compared to 40.0% in the PBO group (P=0.025 and P=0.031, respectively), while no statistically significant difference was noted for the 160 mg group. There were significant differences in the ΔCRP and ΔESR between each XKH004 treatment group and the placebo group.
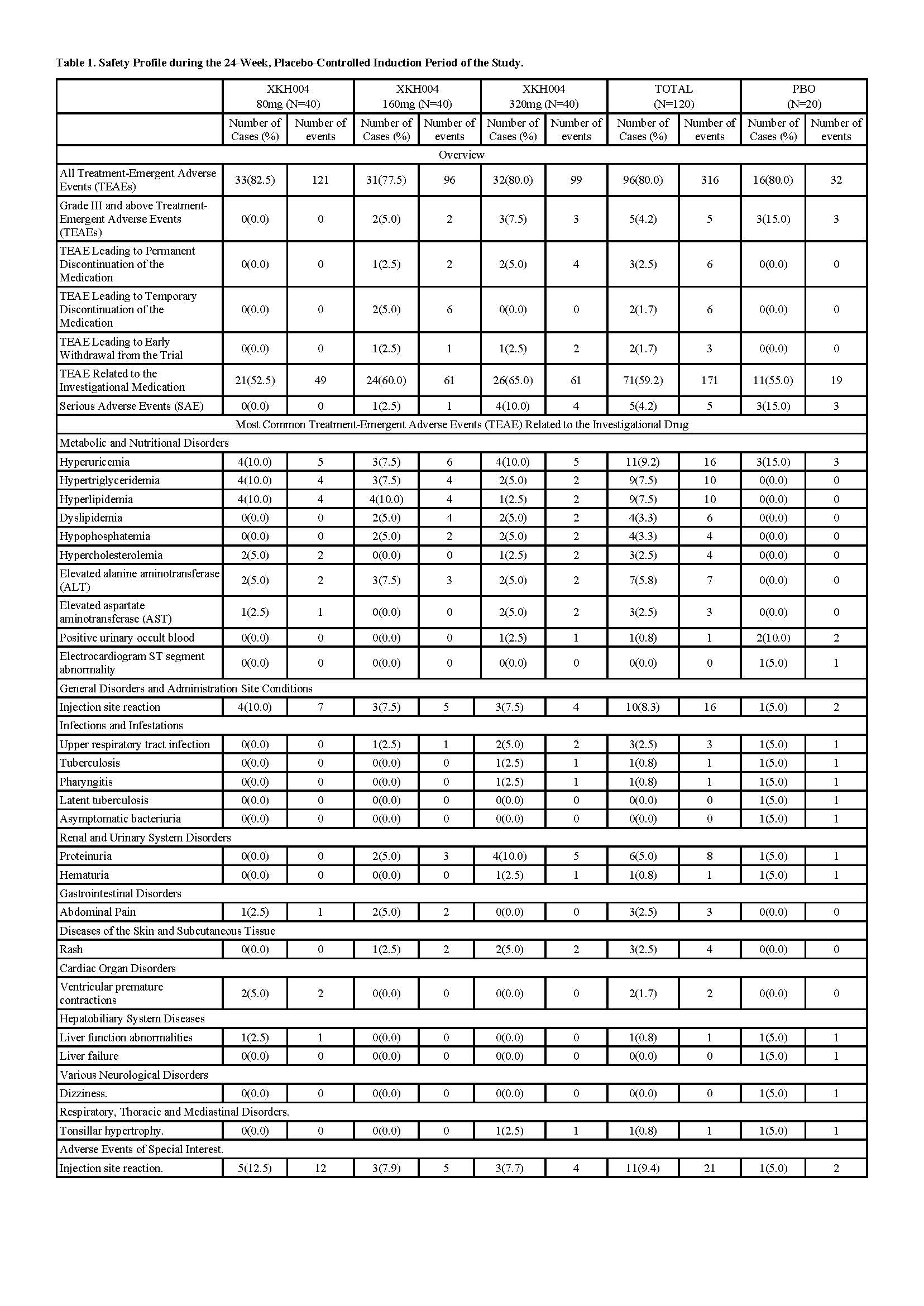
Regarding safety,59.2% patients in the XKH004 group experienced at least one treatment-emergent adverse event related to IMP, showing no statistically significant differences compared to the placebo group(55%) . SAEs were reported in 5 of 120 patients (4.2%) treated with XKH004, while only two SAEs were deemed to be drug-related. In this trial, no immunogenicity risk was observed across all dosage groups.
Conclusion: In patients with active AS, various dosages of XKH004 effectively improved disease activity. The safety and tolerability profile were favorable, and no immunogenicity risk was identified for up to 24 weeks.
To cite this abstract in AMA style:
Zhou L, Zhao J, Sun L, Zhang X, Li X, Hu J, Wu L, Jiang Z, Yang M, Wang X, Mei Y, Huang Q, Chen Y, Li Y, Dong L, Luo H, Guo L, You J, Zhang Y, Ren K, Xu H. Efficacy and Safety of the Recombinant Anti-human IL-17A/F Humanized Monoclonal Antibody Injection (XKH004) in Patients with Active Ankylosing Spondylitis: Findings from a 24-Week, Phase 2 Multicenter, Randomized, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-the-recombinant-anti-human-il-17a-f-humanized-monoclonal-antibody-injection-xkh004-in-patients-with-active-ankylosing-spondylitis-findings-from-a-24-week-phase-2-multicenter/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-the-recombinant-anti-human-il-17a-f-humanized-monoclonal-antibody-injection-xkh004-in-patients-with-active-ankylosing-spondylitis-findings-from-a-24-week-phase-2-multicenter/