Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Olokizumab (OKZ) is a direct inhibitor of interleukin 6, which has shown significant symptom reduction in RA patients, however there is a lack of information regarding the use of olokizumab in real clinical practice. Here we report efficacy and safety of olokizumab in routine practice.
Methods: It was a retrospective 24 weeks observational program in 63 centers including RA patients, who received OKZ (64 mg once every 4 weeks or every 2 weeks subcutaneous). The efficacy was assessed by routine clinical, biochemical parameters, DAS28-ESR, CDAI. Descriptive statistics – mean and standard deviation, absolute and relative frequencies were used. The paired t-test and the chi-square test were used to evaluate the statistical significance. A difference was considered statistically significant at p< 0.05. All AEs were recorded.
Results: 1880 patients with RA were included in the observational program. 1018 of them received OKZ at least 24 weeks by 31.03.2024. Female patients predominated (82.2%). The average age of the patients was 53.2(13.4) years. The average duration of RA was 119.4 (87.9) months. 85.5% of patients had from 1 to 8 comorbid conditions. Most patients were RF (85.6%) and ACCP (81.4%) positive. 330(32.4%) patients had previously taken another bDMARD (tsDMARD) therapy. The average duration of taking the last bDMARD(tsDMARD) was 30.1(32.1) months. Reasons for switching to OKZ included inefficacy (56.7%), non-medical reasons related to the availability of drugs (27.6%), AEs (11.2%), others (4.5%). 57.3% patients were initially received steroids at an average dose of 7.2(4.0) mg/day.
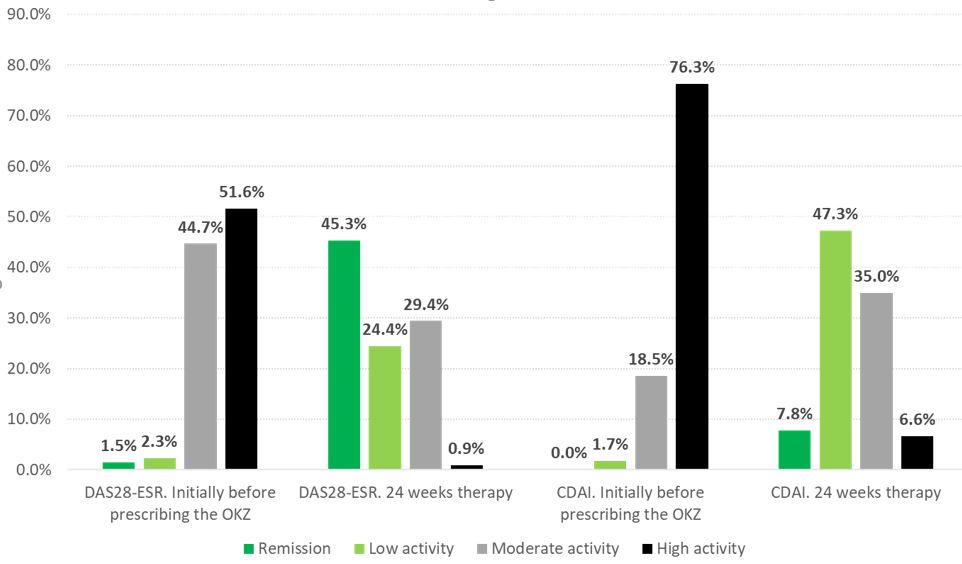
OKZ treatment for 24 weeks led to a significant decrease in the DAS28-ESR and CDAI indices. Remission or low activity according to the DAS28-ESR were achieved by 69.7% of patients, according to the CDAI – by 55.1% of patients (Figure). Number of patients receiving steroid therapy reduced by 53,0% and the average dose decreased to 4.7(2.4) mg/d (compared to baseline p< 0.001).
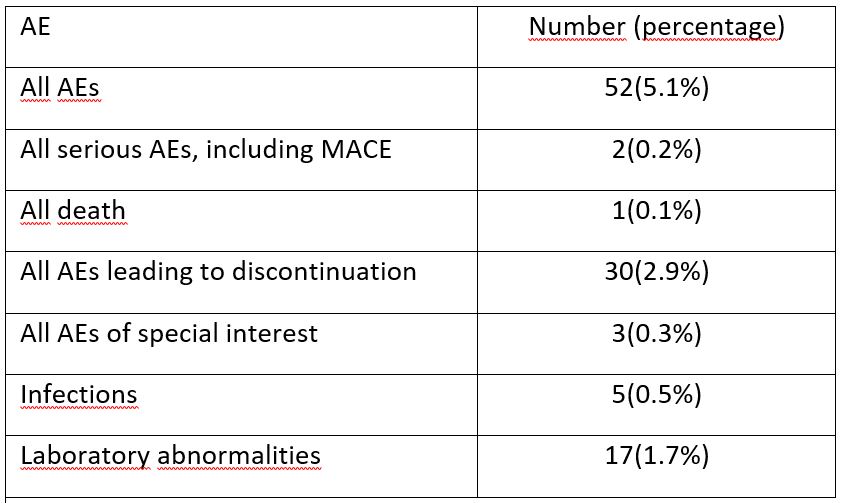
Therapy was discontinued in 27(2.6%) patients due to lack of efficacy. AEs were recorded in 52(5.1%) patients, including MACE in 2(0.2%) patients. AEs led to interruption of OKZ therapy in 30(2.9%) patients (Table).
Conclusion: In real clinical practice olokizumab significantly improves the symptoms of RA, and in some cases allows stopping steroid therapy. Olokizumab did not cause additional safety concerns.
To cite this abstract in AMA style:
Kuzkina S, Zagrebneva A, Togizbayev G, Aitova E, Belozerova N, Patrikeeva I, Samigullina R, Sostak M, Jakovleva E, Chudinov A, Dolgorukova A, Alekseev E, NASONOV E. Olokizumab in Real Clinical Practice: Efficacy and Safety [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/olokizumab-in-real-clinical-practice-efficacy-and-safety/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/olokizumab-in-real-clinical-practice-efficacy-and-safety/