Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To descriptively compare treatment patterns, persistence and effectiveness of upadacitinib (UPA), other JAK inhibitors (JAKi) and tumour necrosis factor inhibitors (TNFi) in rheumatoid arthritis (RA) patients (pts).
Methods: This retrospective, non-interventional, multicenter cohort study used data from the OPAL dataset, derived from electronic medical records. Included pts: aged between 18 and 94 years with a diagnosis of RA, who had initiated for the first time UPA, other JAKi (tofacitinib and baricitinib) or TNFi (adalimumab, certolizumab, etanercept, golimumab, infliximab) between May 2020 and March 2023. Pts were followed up to June 2023. For comparative analyses, pts treated with UPA or other JAKi, and pts treated with UPA or TNFi were 1:1 propensity score matched based on age, sex, line of therapy, DAS28CRP(3) category, index year and combination csDMARD status. Persistence (Kaplan-Meier methods) and clinical effectiveness (DAS28CRP(3), as observed (AO)) are reported.
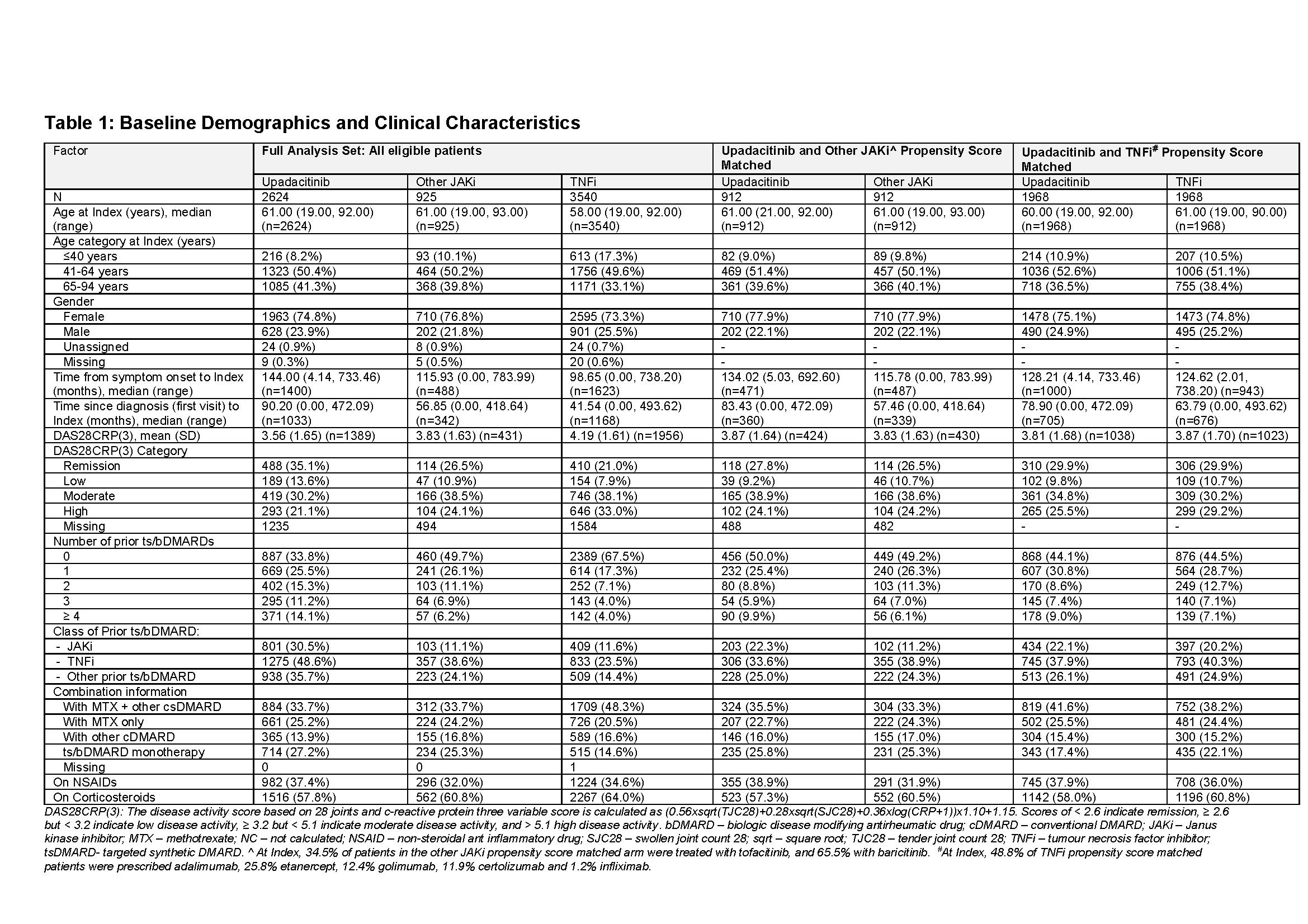
Results: 2,624 pts were initiated with UPA, 925 with other JAKi and 3,540 with TNFi (full analysis set, FAS) (Table 1). In the FAS, pts initiated on UPA and other JAKi were older and had longer time since diagnosis and symptom onset compared to TNFi pts. More pts started TNFi as 1st line therapy (68%) compared to UPA and other JAKi (34% and 50%, respectively). A quarter of UPA and other JAKi pts initiated treatment as monotherapy vs 15% for TNFi.
The matched cohorts consisted of 912 pts each for UPA and other JAKi groups, and 1,968 pts each for UPA and TNFi groups (Table 1). Both cohorts were similar for age, gender and disease severity at index. Some differences remained, including time since diagnosis, number of prior b/tsDMARDs and concomitant treatments.
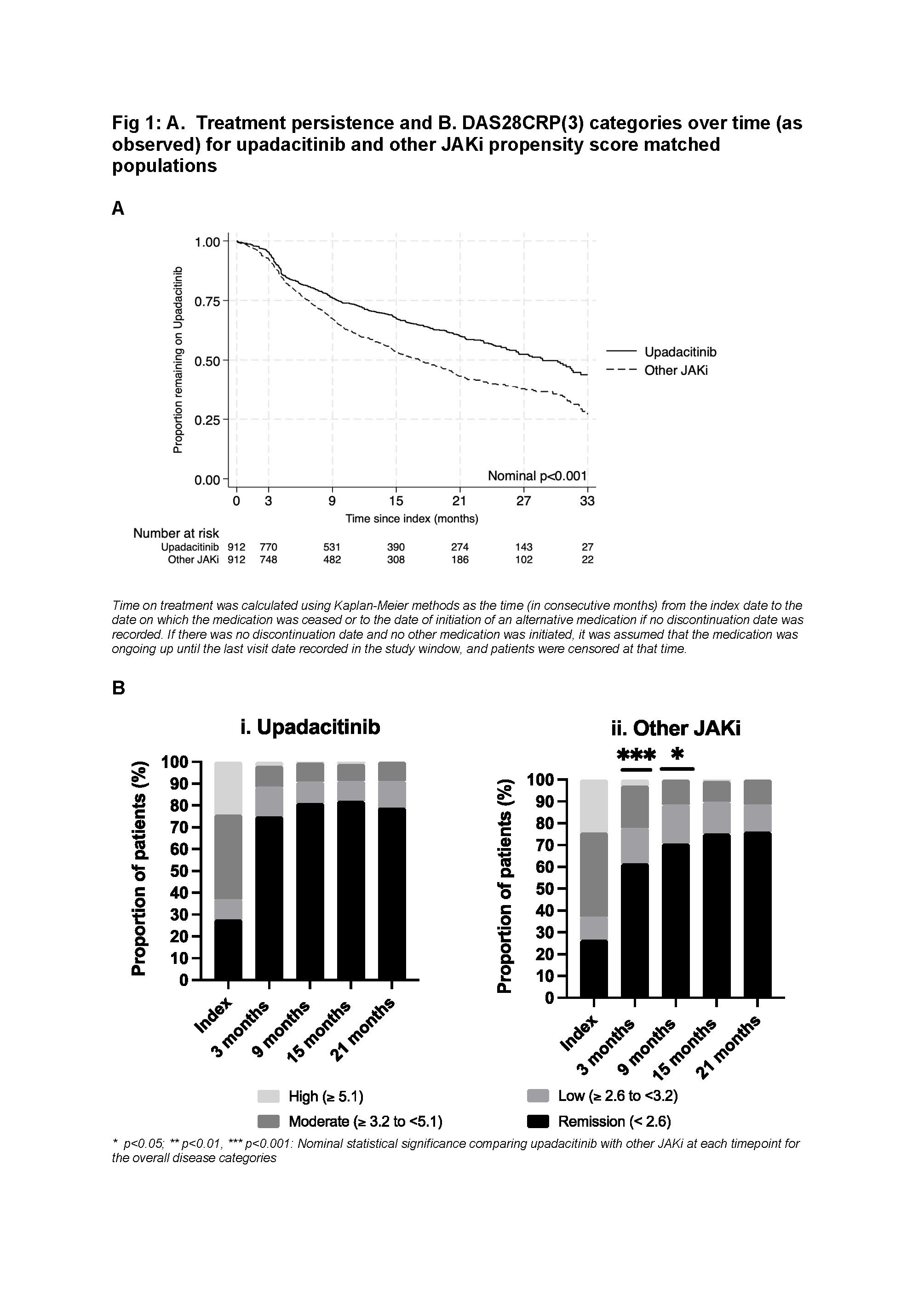
In the UPA/other JAKi matched cohort, median time on treatment for UPA was greater than other JAKi (28.8 months [95% CI 25.6 – 32.4 months] vs 17.2 months [95% CI 14.9 – 19.8 months], p< 0.001). 67% and 60% UPA pts remained on therapy at 15 and 21 months respectively vs 53% and 43% for other JAKi. This was consistent regardless of line of therapy, or if initiated as monotherapy or as combination therapy. In the overall matched cohort, more UPA pts achieved DAS28CRP(3) remission at 3 months compared to other JAKi (UPA, 75% [index 28%] vs other JAKi, 61.5% [index 26.5%]) (Fig 1).
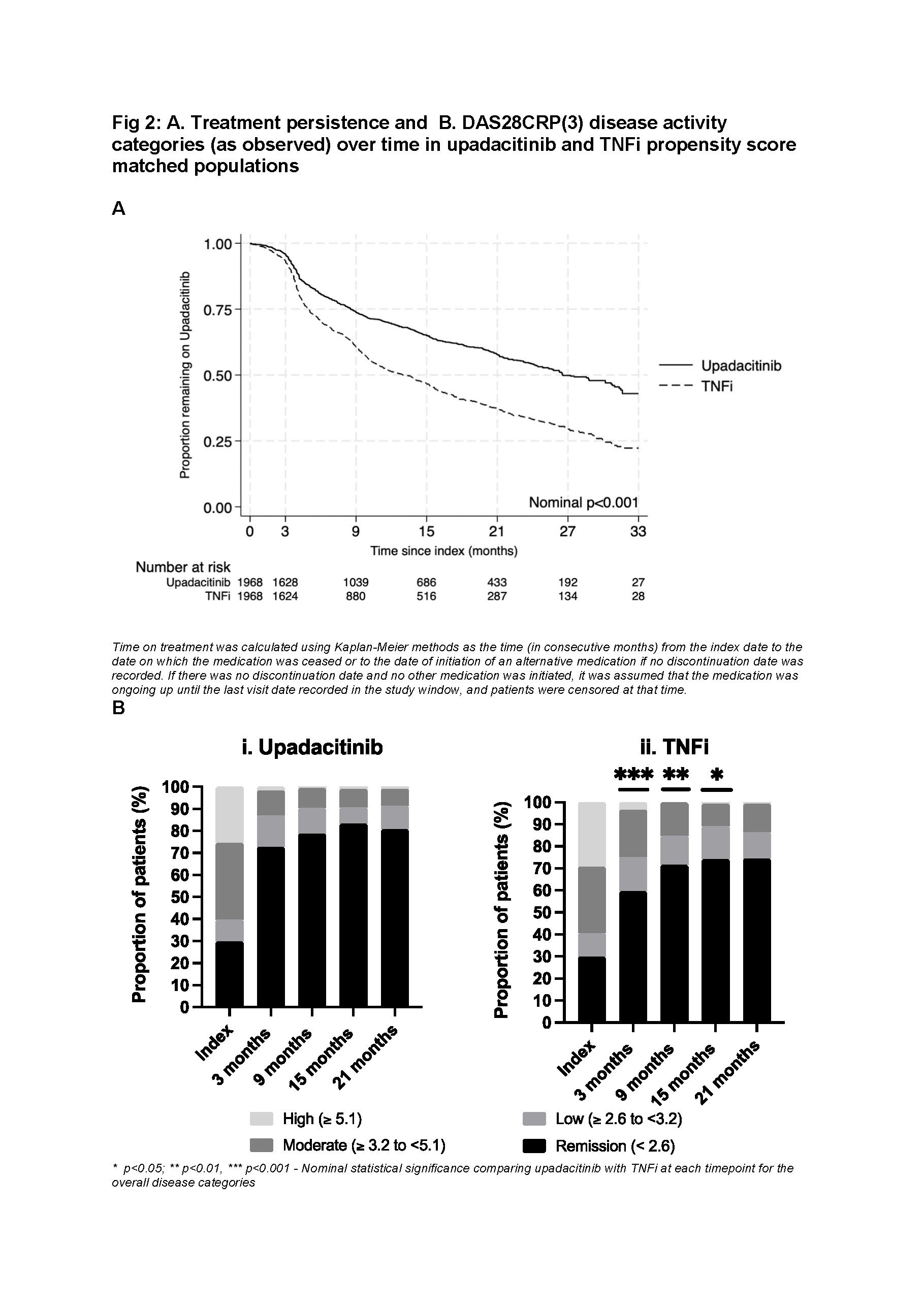
In the UPA/TNFi matched cohort, median time on treatment for UPA was greater than TNFi (26.6 months [95% CI 24.85 – 30.8 months] vs 13.3 months [95% CI 11.5 – 14.5 months], p< 0.001). 65% and 58% UPA pts remained on therapy at 15 and 21 months respectively vs 47% and 37% for TNFi. This was consistent regardless of line of therapy, or if initiated as monotherapy or combination therapy. In the overall matched cohort, more UPA pts achieved DAS28CRP(3) remission at 3 months compared to TNFi (UPA, 73% [index 30%] vs TNFI, 59.5% [index 30%]) (Fig 2).
Conclusion: In real-world RA pts, UPA treatment showed significantly longer persistence compared to TNFi and JAKi, irrespective of line of therapy or if used as monotherapy or combination therapy. UPA pts also had greater rates of remission at 3 months compared to TNFi and other JAKi. Overall, this suggests a favourable UPA clinical profile in RA patients.
To cite this abstract in AMA style:
Ciciriello S, Youssef P, Tahir T, Smith T, O'Sullivan C, Leadbetter J, Butcher B, Calao M, Walsh N, Littlejohn G. Upadacitinib vs TNFi and Other JAKi Treatment Outcomes in Australian Rheumatoid Arthritis Patients: Descriptive Comparison of Persistence and Effectiveness Using the OPAL Dataset [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/upadacitinib-vs-tnfi-and-other-jaki-treatment-outcomes-in-australian-rheumatoid-arthritis-patients-descriptive-comparison-of-persistence-and-effectiveness-using-the-opal-dataset/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-vs-tnfi-and-other-jaki-treatment-outcomes-in-australian-rheumatoid-arthritis-patients-descriptive-comparison-of-persistence-and-effectiveness-using-the-opal-dataset/