Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In patients taking opioid analgesics, opioid-induced constipation (OIC) is estimated to affect between 41% to 80% of patients and is caused by peripheral mu-opioid receptor activation in the gastrointestinal (GI) tract. Methylnaltrexone is a peripherally-acting mu-opioid receptor antagonist that reverses opioid-related constipating effects in the GI tract without affecting centrally-mediated analgesia. Patients with a rheumatic disease may take opioid analgesics for chronic pain and develop OIC. The aim of this subgroup analysis was to assess the efficacy/safety of methylnaltrexone for OIC in patients who participated in a phase 3 trial and had ankylosing spondylitis (AS), fibromyalgia, rheumatoid arthritis (RA), or osteoarthritis (OA).
Methods: A phase 3, randomized, double-blind, placebo-controlled trial enrolled adults with chronic (≥2 months) noncancer pain taking ≥50 mg oral morphine equivalent dose (MED) for ≥2 weeks who had a mean of < 3 bowel movements/week. Enrolled patients with a medical history of AS, fibromyalgia, RA, or OA who received subcutaneous (SC) methylnaltrexone 12 mg every other day or placebo for 4 weeks were included in the current analysis. The original trial coprimary endpoints were the percentage of patients with a rescue-free bowel movement (RFBM; ie, bowel movement occurring without any laxative use within previous 24 hours) within 4 hours after the first treatment was administered and the percentage of treatments (injections) resulting in an RFBM within 4 hours during the 4-week treatment period.
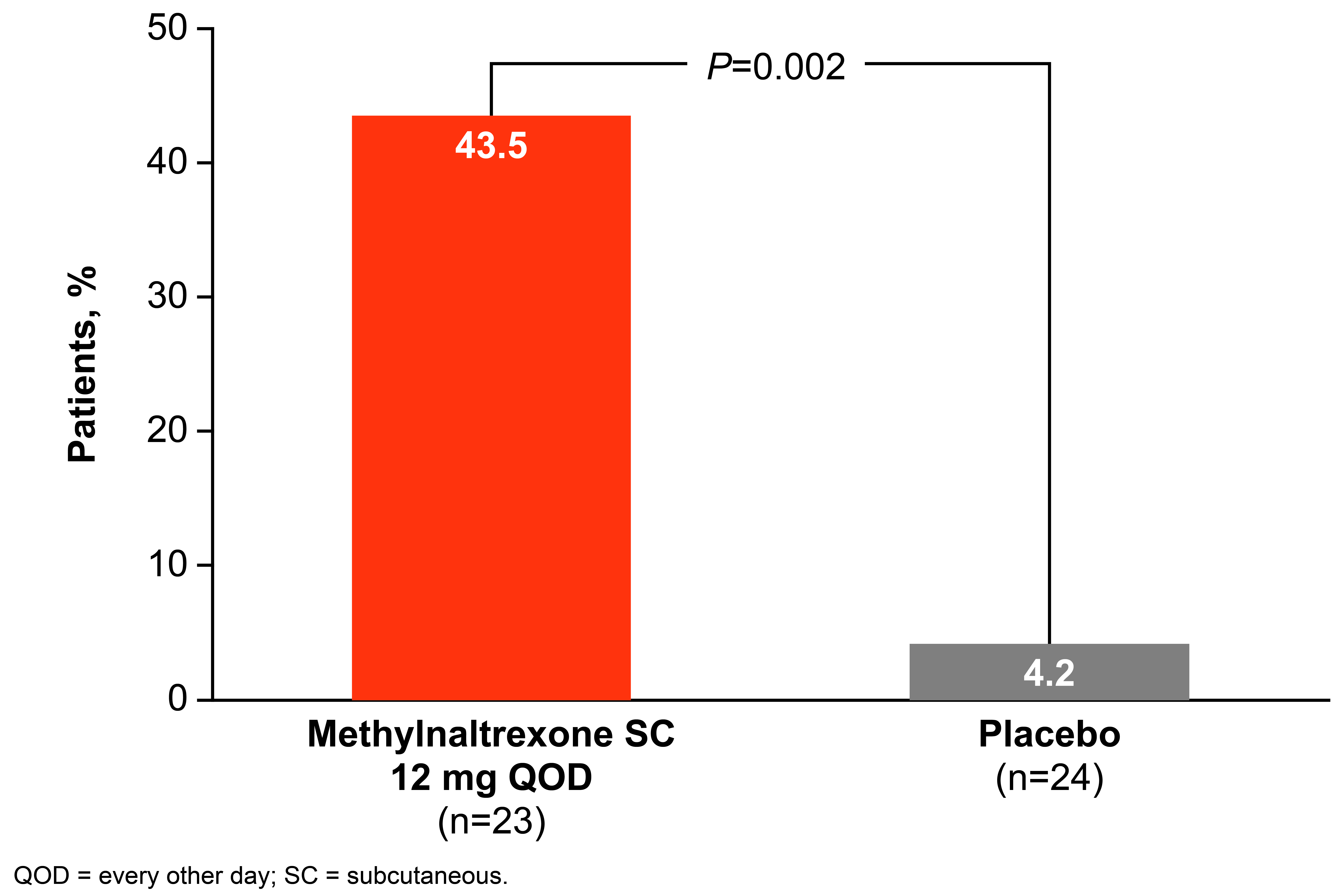
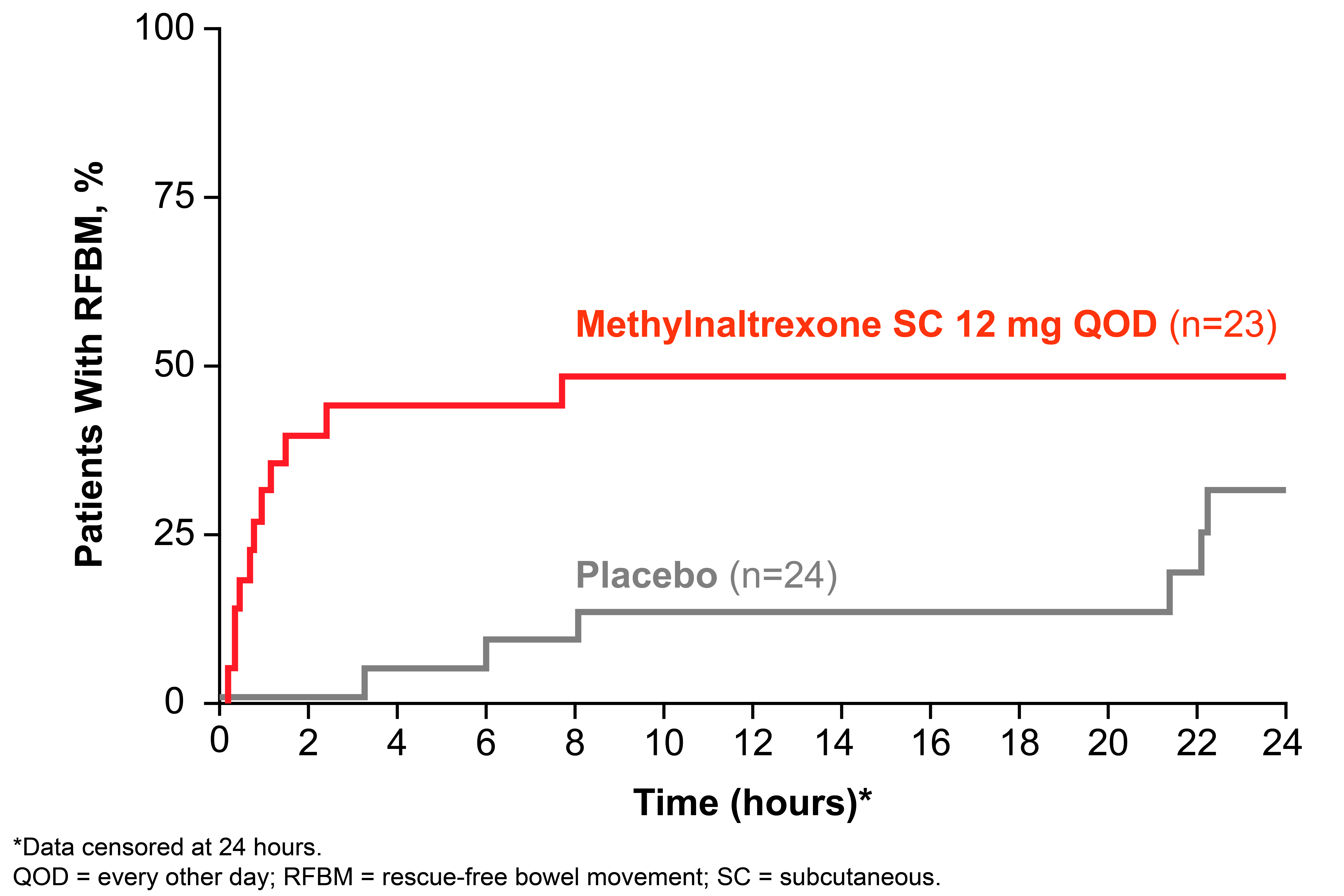
Results: A total of 23 and 24 patients were included in the methylnaltrexone and placebo groups, respectively: the mean (SD) age was 50.6 (8.8) y vs 54.0 (11.8) y; the majority was female (82.6% vs 75.0%); the baseline mean (SD) weekly number of RFBM was 0.8 (0.6) vs 1.1 (0.7); osteoarthritis (43.5% vs 58.3%) and fibromyalgia (39.1% vs 33.3%) were the most common conditions. The percentage of patients taking a morphine equivalent ≥100 mg at baseline was 56.5% and 45.8% in the methylnaltrexone and placebo groups, respectively. Overall, significantly more patients treated with methylnaltrexone 12 mg QOD had an RFBM within 4 hours of the first dose compared with placebo (Figure 1; P=0.002), with a rapid onset of action (Figure 2). In addition, a significantly higher percentage of treatments (injections) during the 4-week period resulted in an RFBM within 4 hours with methylnaltrexone vs placebo (28.7% vs 13.5%, respectively; P=0.01). One patient treated with methylnaltrexone discontinued from the study due to an adverse event (AE). Common AEs were mostly GI-related, possibly due to symptoms of OIC and/or mechanism of induction of an RFBM. The most common AEs in the methylnaltrexone group (n=23) vs placebo (n=24) were: diarrhea (17.4% vs 8.3%), abdominal pain (13.0% vs 16.7%), headache (8.7% vs 4.2%), hot flush (8.7% vs 8.3%), nausea (8.7% vs 4.2%), upper abdominal pain (8.7% vs 0%), urinary tract infection (8.7% vs 4.2%), and flatulence (4.3% vs 8.3%).
Conclusion: Subcutaneous methylnaltrexone QOD was efficacious, with a rapid onset of action, and generally well tolerated for the treatment of OIC in adults with rheumatic disease.
To cite this abstract in AMA style:
Moreland L, Brookfield R, Laitman A. Subcutaneous Methylnaltrexone Treatment of Opioid-Induced Constipation in Adults with Rheumatic Conditions [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/subcutaneous-methylnaltrexone-treatment-of-opioid-induced-constipation-in-adults-with-rheumatic-conditions/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-methylnaltrexone-treatment-of-opioid-induced-constipation-in-adults-with-rheumatic-conditions/