Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: arpal tunnel syndrome (CTS) is the most common peripheral neuropathy secondary to chronic median nerve compression at the wrist within the space-limited carpal tunnel (CT). CTS management includes hand therapy, splinting, corticosteroid injection, and surgical decompression. This IRB-approved study investigated the safety and complications of CT injections.
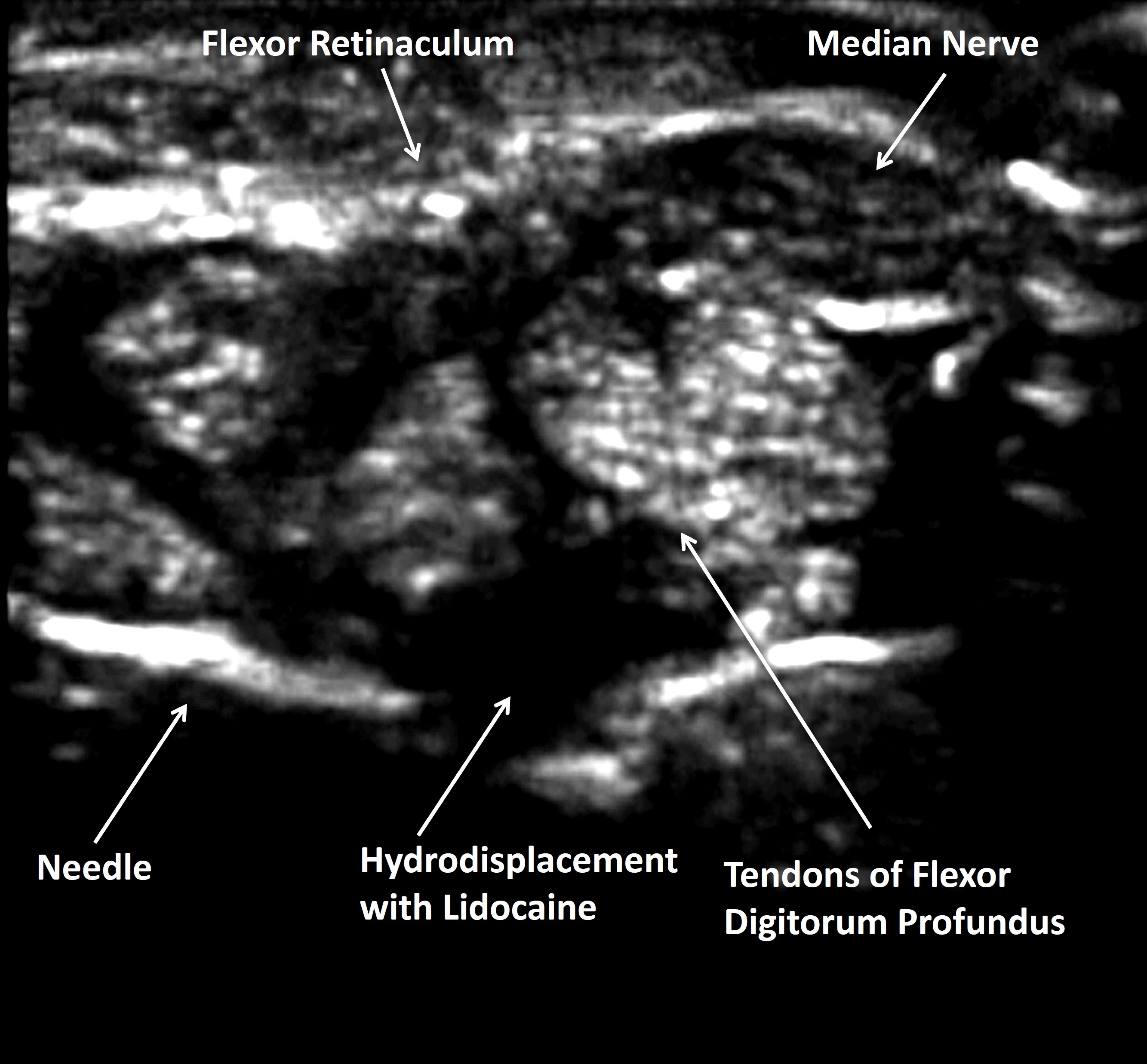
Methods: From a procedural database the outcomes of 1089 consective CT injections were reviewed. Patients underwent CT injection for clinical CTS symptoms, including numbness/tingling in the distribution of the median nerve, hand grip weakness, thenar muscle atrophy, and/or positive Tinel’s or Phalen’s sign. All subjects were offered CT surgery, and those that refused received CT injections. The CT injection technique was the traverse ulnar technique, where the 5/8-inch 25 g needle was introduced on the ulnar side of the volar wrist (towards the midline away from the ulnar artery) and directed radially until the needle tip resided below the tendons of the flexor digitorum superficialis and flexor digitorum profundus tendons directly below the median nerve. 3 ml of lidocaine was injected to create a fluid space and then using a syringe exchange 40 mg triamcinolone acetonide was injected through the same needle below the superficialis and profundus bundles. In subjects referred for CT surgery, success was defined as no recurrent symptoms and no further CT injections or other therapy.
Results: The 183 subject cohort was 84.7% female with the diagnoses of inflammatory arthritis (55.2%), occupational CTS (30%), diabetes mellitus (10.9%), and hypothyroidism (3.8%). The 183 subjects underwent a total of 1089 CTS injections (mean 6.0± 9.5 injections/subject). 57.9% injections were on the right CT and 42.1% were on the left CT. 30.8% underwent a single session of CT injection, and 69.2% underwent multiple injection sessions because of recurrent symptoms. In those who had multiple injection sessions, the average interval between injection sessions were 10.6± 11.0 months. Complications occurred in 0.82% (9/1089) of CT injections: 0.18% (2/1089) tendon ruptures, 0.09% (1/1089) infection with Staphylococcus epidermidis, and 0.55% (6/1089) with reversible skin atrophy. There were no hematomas, vascular injury, or nerve injuries. In the 2 subjects (1 Rheumatoid arthritis, 1 Diabetes mellitus) with tendon rupture each had received 6 CTS injections. 21.3% (39/183) of subjects eventually underwent CT release surgery. CT surgery was successful in 71.8% (28/39) subjects. However, CT surgery failed in 28.2% who experienced recurrent symptoms and required additional postsurgical injections, splinting, or occupational therapy.
Conclusion: CT injections using the transverse ulnar approach are safe and effective with a low risk of complications. CT surgical release in recurrent cases provides lasting relief to many patients, but also fails in a substantial minority (28.2%) who then require reinjection or other non-surgical therapy.
To cite this abstract in AMA style:
sibbitt w, Fatma d, Emil N, Muruganandam M, Fields R, Adarsh V, O'Sullivan F, Hattori S, Delgado F, Tapia K. The Outcomes of Carpal Tunnel Injections and Carpal Tunnel Surgery [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-outcomes-of-carpal-tunnel-injections-and-carpal-tunnel-surgery/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-outcomes-of-carpal-tunnel-injections-and-carpal-tunnel-surgery/