Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Nonadherence to hydroxychloroquine (HCQ) is highly prevalent in lupus (or SLE) and two-fold higher in patients of Black race or Hispanic ethnicity. While HCQ nonadherence risks 45% higher acute care visits and potentially drives disparities, regular use of the recommended HCQ dose (< 5 mg/kg/d) is still associated with 6-fold higher acute care visits due to subtherapeutic levels. This conundrum could be solved by therapeutic HCQ blood level monitoring as it can guide clinicians to optimize HCQ dosing at individual patient level to maximize efficacy & adherence thereby reducing flares & acute care visits. However, to ensure universal use of therapeutic HCQ blood level monitoring, we need to establish: a) its effectiveness in reducing acute care use in SLE, particularly in patients of Black race or Hispanic ethnicity, who historically face higher nonadherence & worse outcomes; b) patients’ acceptability to undergo HCQ level testing.
Methods: Patients with SLE without contraindications to HCQ were offered HCQ blood level testing during consecutive clinic visits. HCQ blood levels (ng/ml) were categorized as: a) subtherapeutic (< 750), b) therapeutic (750-1200), or c) supratherapeutic ( >1200) (Garg, AC&R 2024). All SLE-related initial and recurrent acute care (hospital or ER) visits from the index clinic visit through the next clinic visit were manually abstracted. Covariates included socio-demographics, SLE disease activity score, SLE damage index, kidney function, steroid & HCQ doses, history of previous acute care visits, exposure to SLE triggers, & immunosuppressive drugs. Using Proportional Means Hazards models, we first examined associations between HCQ blood levels and recurrent acute care visits. Using our hazards model, we then calculated the predicted mean frequency of acute care visits with therapeutic HCQ levels stratified by race adjusting for covariates. Finally, to estimate patients’ acceptability to undergo therapeutic HCQ level monitoring, we calculated percentage of eligible patients undergoing HCQ blood draws.
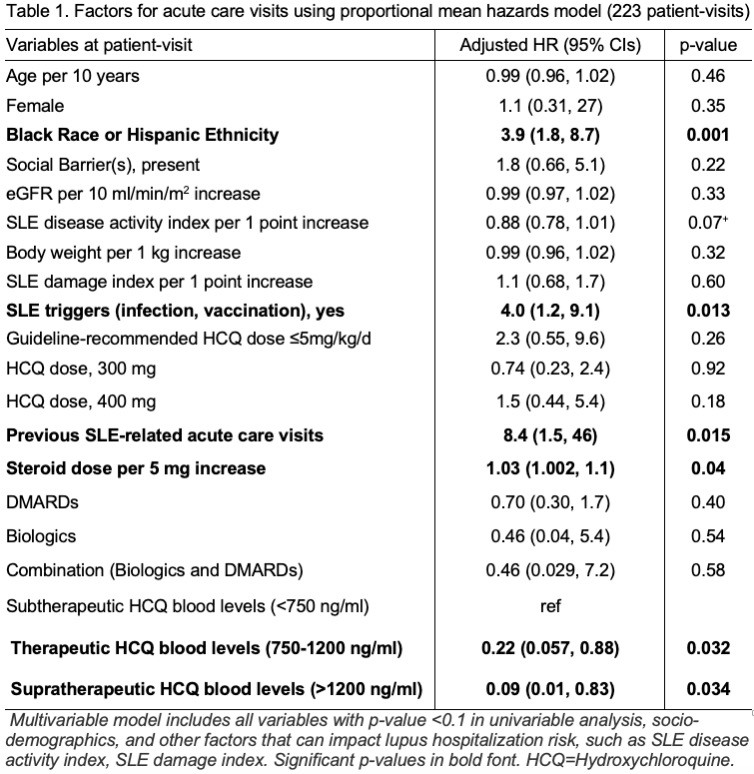
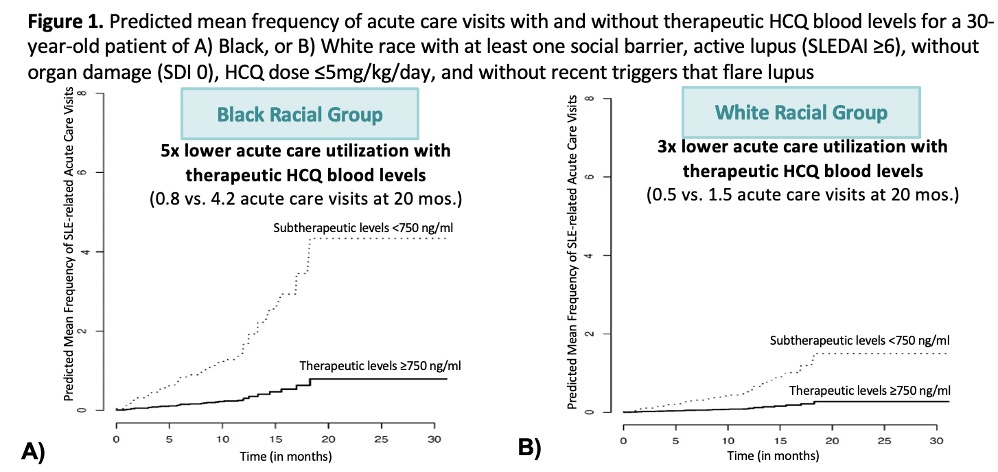
Results: Across 223 patient-visits, a total of 39 SLE-related acute care visits were observed. We noted that patients of Black race or Hispanic ethnicity had 4-fold higher risk of acute care visits (Adjusted HR 3.8; Table 1). Achieving therapeutic HCQ blood levels (750-1200) was associated with a 78% lower risk of acute care visits compared to those with subtherapeutic levels, < 750 ng/ml (Table 1). The predicted mean frequency of SLE-related acute care visits with therapeutic HCQ levels for a 30-year-old person of Black race with social barriers, active lupus, no organ damage, 5mg/kg dosing, and no exposure to SLE triggers was five times lower compared to a patient with similar characteristics but with subtherapeutic levels (0.8 vs. 4.2 visits at 20 mos.; Figure 1). A high completion rate of 93% indicated high patient acceptability and interest to undergo HCQ level testing.
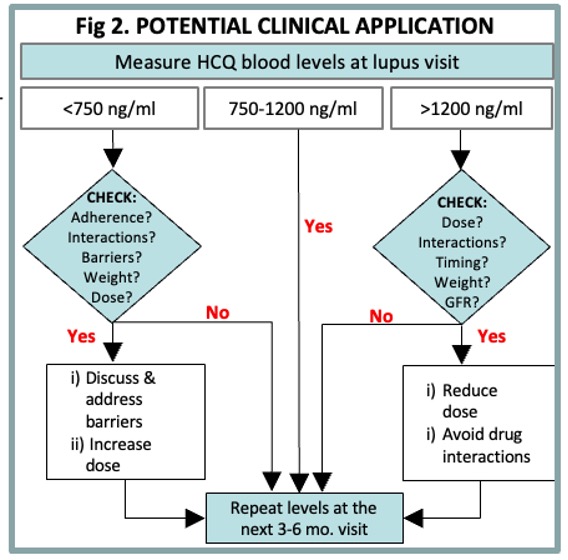
Conclusion: Therapeutic HCQ blood levels (750-1200 ng/ml) lowered acute care visits by 78%, and predicted 5x lower acute care visits in patients of Black race or Hispanic ethnicity. The proposed therapeutic HCQ level monitoring workflow (Fig. 2) can be used during visits to improve outcomes & reduce disparities in SLE.
To cite this abstract in AMA style:
Garg S, Valiente G, Saric C, Chewning B, Bartels C. Therapeutic Hydroxychloroquine Blood Levels Predict Lower Mean Frequency of Recurrent Acute Care Utilization in Lupus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/therapeutic-hydroxychloroquine-blood-levels-predict-lower-mean-frequency-of-recurrent-acute-care-utilization-in-lupus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/therapeutic-hydroxychloroquine-blood-levels-predict-lower-mean-frequency-of-recurrent-acute-care-utilization-in-lupus/