Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
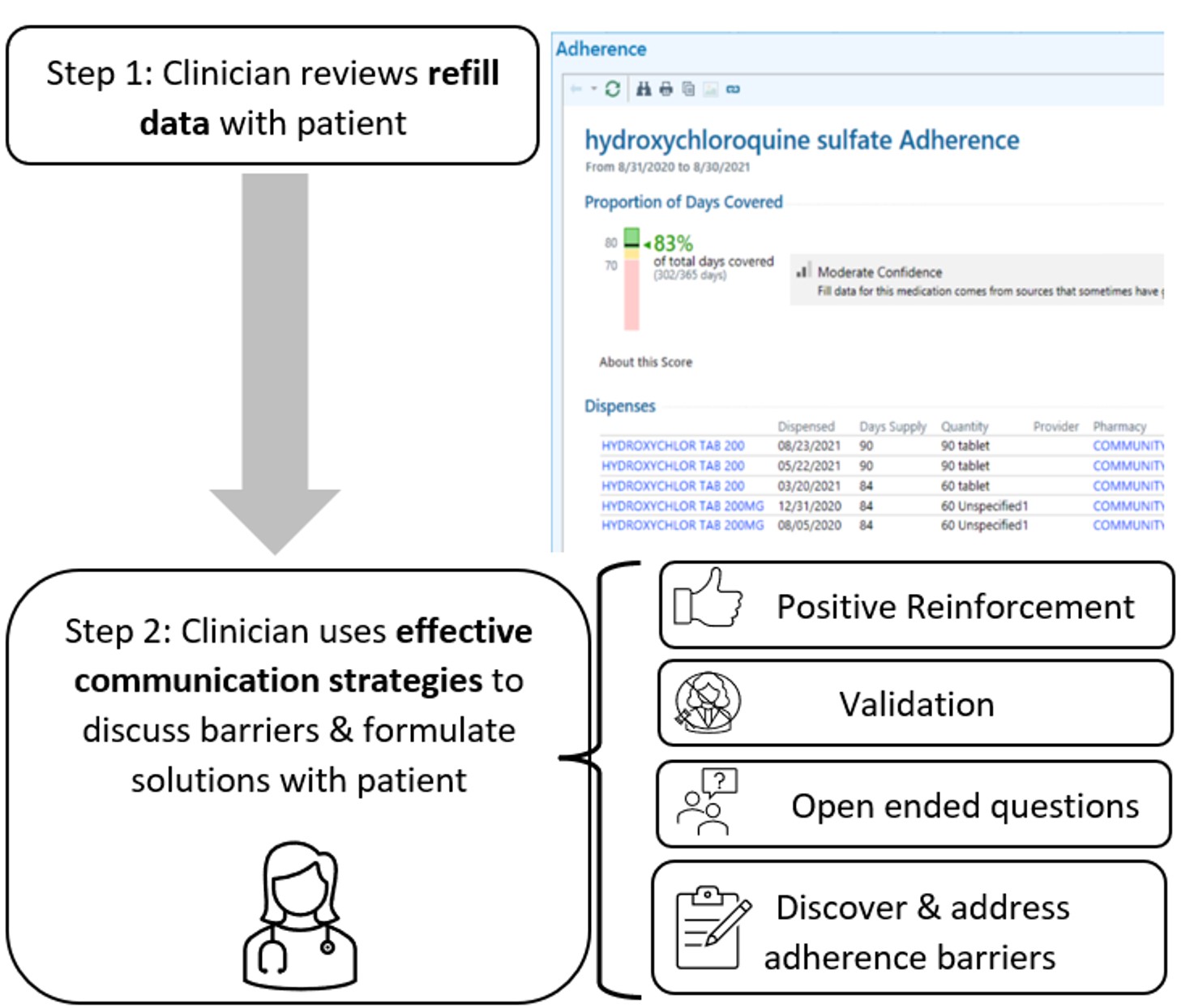
Background/Purpose: Medication nonadherence is as high as 75% among patients with rheumatic diseases, leading to increased morbidity, mortality, and cost. Existing adherence interventions tend to be resource intensive. We developed an adherence intervention for patients with SLE that was feasible, acceptable, and effective. The 2-step intervention involves the clinician: 1) reviewing pharmacy refill data with the patient; and 2) using specified communication strategies to discuss adherence barriers and formulate solutions with the patient (Figure 1). In this study, we adapted the intervention for general rheumatology and evaluated its feasibility, acceptability, and effect on clinicians’ adherence practices and perceptions.
Methods: We recruited clinicians from an academic rheumatology practice. We trained clinicians on the adherence intervention, and they delivered the intervention for 4 weeks with all return patients prescribed long-term rheumatic disease medications. We collected clinician surveys at baseline and post-intervention on a 5-point Likert scale. We also collected patient satisfaction surveys during the intervention period.
Results: Six clinicians participated (5 attending physicians, 1 Nurse Practitioner), and 5 completed baseline and follow up surveys.
At follow up, clinicians reported high scores for the intervention’s feasibility (4.8/5), acceptability (4.5/5), and appropriateness (4.4/5). Clinicians also expressed high likelihood to recommend the intervention to a colleague (4.2/5) and continuing to use the intervention in the future (4.2/5).
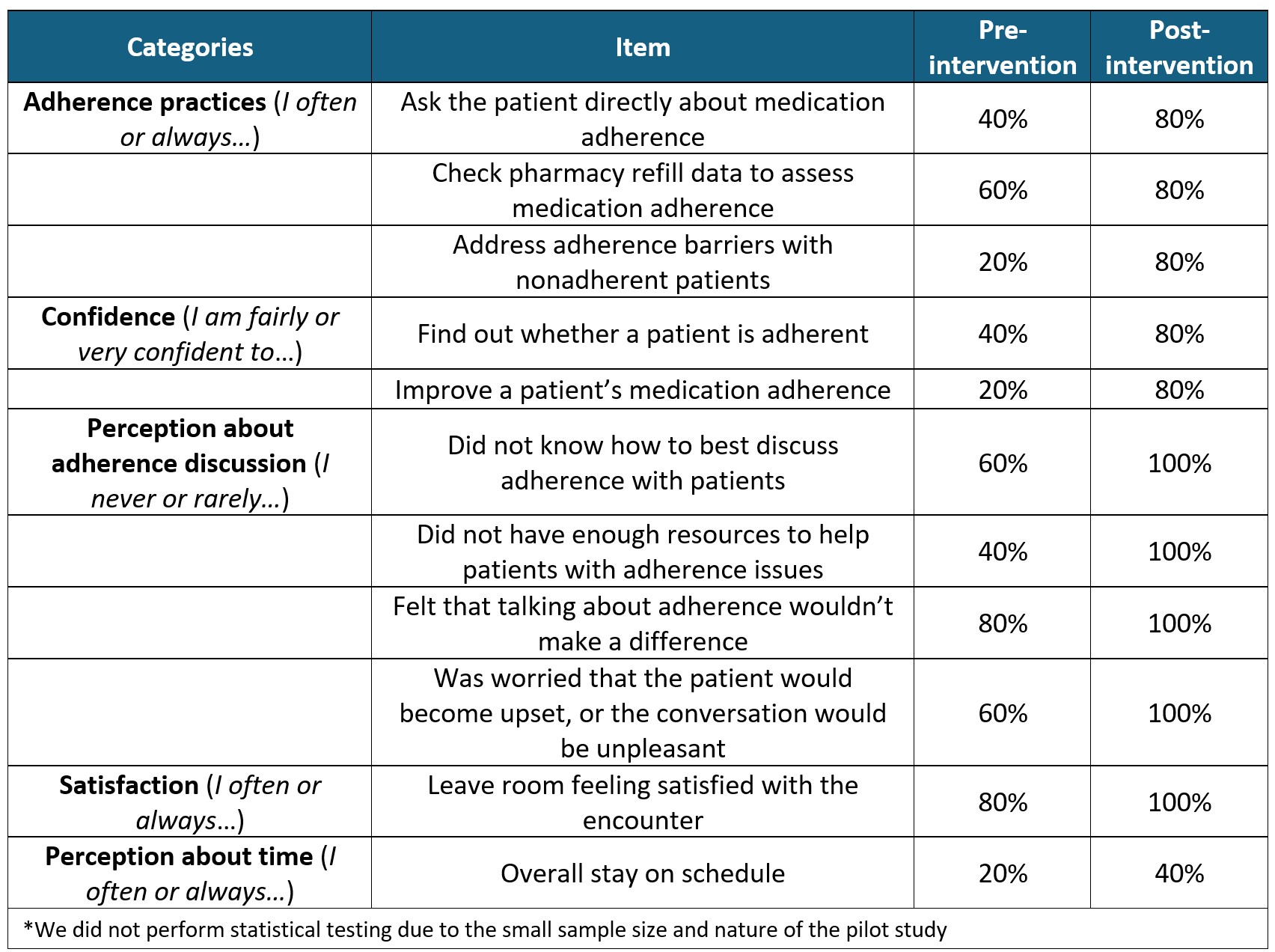
Additionally, compared to pre-intervention, clinicians reported being more likely to ask patients about adherence, check pharmacy refills, and address adherence barriers post-intervention (Table 1). They also felt more confident to screen for nonadherence and explore ways to improve adherence. Most reported knowing how to discuss adherence, having enough resources to help improve adherence, and not worrying that adherence discussions would be futile or unpleasant. Lastly, clinicians reported being more likely to leave the room feeling satisfied and staying on schedule.
Among the 179 eligible patients seen during the intervention period, 82 (mean age 56, 84% female, 70% White, 27% Black; 4% Hispanic) with 15 rheumatic diagnoses completed post-intervention surveys. Patients most commonly reported feeling determined (29%), empowered (24%), inspired (18%), proud (16%), and excited (9%) after the intervention visit.
Conclusion: The adherence intervention was feasible and acceptable to general rheumatology clinicians and patients. Our results also suggest that the intervention was able to change clinicians’ adherence practices, improve confidence level and perception about adherence discussions. Future work will determine the intervention’s effect on medication adherence, test and implement the intervention in a larger sample of clinicians and assess its sustainability.
To cite this abstract in AMA style:
Sun K, Carnago L, Smith I, Andonian B, Balevic S, Shah A, Sims C, Pollak K, Corneli A, Bosworth H, Clowse M. Pilot Intervention to Improve Medication Adherence Among Patients with Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pilot-intervention-to-improve-medication-adherence-among-patients-with-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pilot-intervention-to-improve-medication-adherence-among-patients-with-rheumatic-diseases/