Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: While axial spondyloarthritis (AxSpA) was historically perceived as a “white man’s disease”, it is now appreciated as a condition that can affect individuals of any sex, race, or ethnicity. Enrolling diverse cohorts of participants in drug trials is crucial to ensure that the results are applicable to all patients with axSpA.
Methods: We searched the ClinicalTrials.gov database for the condition ‘AxSpA’, including ankylosing spondylitis/radiographic axSpA (AS/r-axSpA) and non-radiographic axSpA (nr-axSpA). We selected all completed phase 2, 3, and 4 drug trials with adult participants and results posted. We retrieved data on sex, race, ethnicity, trial location and characteristics, and performed descriptive statistics using Excel.
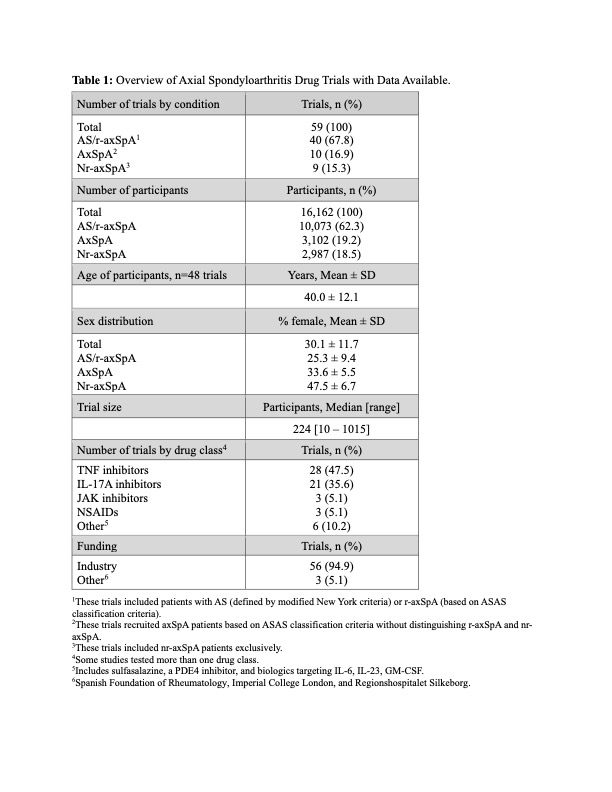
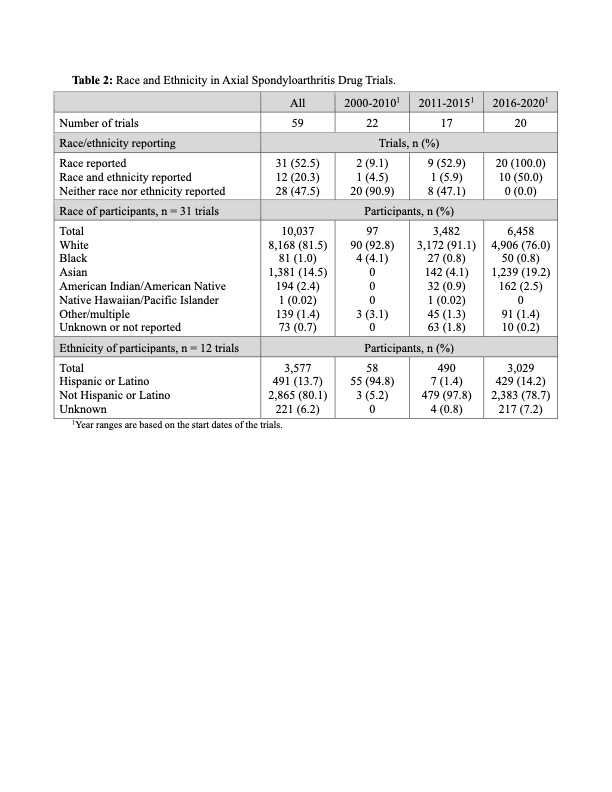
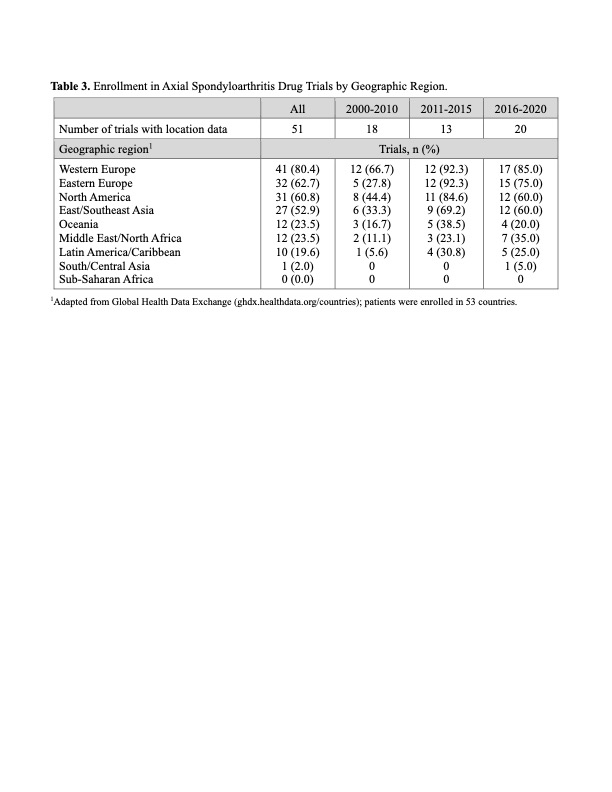
Results: A total of 59 drug trials with 16,162 participants were analyzed: 40 trials in AS/r-axSpA, 10 in axSpA, and 9 in nr-axSpA (Table 1). These trials had start dates between 2003 and 2020 and investigated the performance of TNF inhibitors (28 trials), IL-17A inhibitors (21 trials), JAK inhibitors (3 trials), NSAIDs (3 trials), and others (6 trials). 56/59 trials were industry-funded. Trial sizes varied, with a median enrollment of 224 participants (range 10 to 1015). The average age of participants, based on data from 48 trials, was 40.0 ± 12.1 years (mean ± SD). Females constituted 30.1 ± 11.7 % of participants across all trials, with 25.3 ± 9.5 % females in AS/r-axSpA trials, 33.6 ± 5.8 % in axSpA trials and 47.5 ± 7.2 % in nr-axSpA trials. Among all 59 trials, 31 (52.5%) reported race, 12 (20.3%) reported race and ethnicity, and 28 (47.5%) reported neither (Table 2). Race reporting increased from 9.1% (2000-2010) to 52.9% (2011-2015) and 100% (2016 -2020). Of the 10,037 participants with race data, 81.5% were White, 1.0% Black, 14.5% Asian, 2.4% American Indian/Alaska Native (AI/AN), and 0.02% Native Hawaiian/Pacific Islander (NH/PI). Excluding earlier studies due to low sample size, Asian representation increased from 4.1% (2011-2015) to 19.2% (2016-2020), while Black representation was consistently low at 0.8%, and AI/AN increased from 0.9% to 2.5%. In studies reporting ethnicity, 13.7% of subjects were Hispanic or Latino, increasing from 1.4% (2011-2015) to 14.2% (2016-2020). Across 51 trials with location data, study participants were recruited in 53 countries (Table 3); 80.4% of trials recruited in Western Europe, 62.7% in Eastern Europe, 60.8% in North America, 52.9% in East/Southeast Asia, 23.5% in Oceania, 23.5% in Middle East/North Africa, 19.6% in Latin America/Caribbean, 2.0% in South/Central Asia, and none in Sub-Saharan Africa.
Conclusion: Ensuring diversity in clinical trials is vital for the generalizability of results. Our analysis of axSpA drug trials conducted over the last two decades shows that enrollment of women largely reflects the demographics of the disease. The reporting of race and ethnicity has improved over time. While representation of Asians and AI/AN has increased in recent years, there is persistent underrepresentation of Blacks and NH/PI. Efforts to enhance diversity in axSpA drug trials should continue, focusing on inclusive recruitment strategies and expanding recruitment in underrepresented regions globally.
To cite this abstract in AMA style:
Choufani M, Ghusn W, Ermann J. Diversity in Axial Spondyloarthritis Drug Trials: Examining Enrollment by Sex, Race, Ethnicity and Geographic Region [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/diversity-in-axial-spondyloarthritis-drug-trials-examining-enrollment-by-sex-race-ethnicity-and-geographic-region/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diversity-in-axial-spondyloarthritis-drug-trials-examining-enrollment-by-sex-race-ethnicity-and-geographic-region/