Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In developed countries, the impact of healthcare systems on the environment is sizeable (roughly 8% of the carbon footprint). So, it is essential to incorporate a medico-ecological approach into our practices and prescriptions. In rheumatology, there are now numerous biosimilars for the same biological DMARD (Disease-modifying antirheumatic drugs). We are questioning whether there may be a medico-ecological advantage of one over the others.
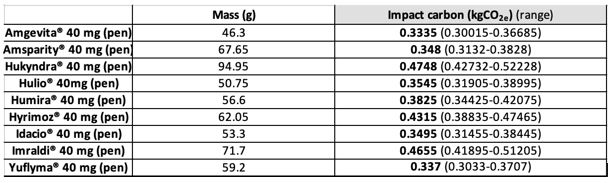
The aim of this study was to evaluate and compare the carbon footprint (CO2e) of the different adalimumab 40mg pens. We focused solely on the container, excluding the active ingredient. The pen and its packaging were taken into account. We also studied 2 syringes
Methods: In this descriptive study, the life cycle of the original drug and its eight 40 mg biosimilar drugs were analysed. The Ansys Granta EduPack® software was used following the “eco-audit” method proposed by Ashby. The resulting emissions correspond to the entire lifespan of the different factors from material extraction to their disposal, consisting of production and transportation. The amount of water needed for manufacturing and their recyclable proportions has also been assessed.
Results: We have found that the biosimilar with the greatest impact represents almost +40% equivalent of CO2 more than the one with the lowest impact. We have observed that one injection by syringe represents 2 to 3 times fewer emissions than a pen. There was significant variability in water quantity that is necessary to produce these drugs, ranging from a single unit to six times as much. Also, there are disparities in recyclable content.
Conclusion: A year of treatment using an adalimumab pen for one patient represents at least 9 kg CO2e (excluding the active ingredient). In France, in 2022, the greenhouse emissions related to 40mg adalimumab pens represent 348 tons CO2e, the equivalent of 2.2 million kilometres by plane.
In conclusion, there are substantial disparities in carbon emissions among different adalimumab biosimilar drugs. Comparatively, the syringe halves the emissions compared to the pen. Our results are expected to help both manufacturers and prescribers decarbonize healthcare.
To cite this abstract in AMA style:
Moninot I, CHAPURLAT R, FEURER E, Cottinet P, Le M, Fontana A. What Is the Carbon Footprint of Adalimumab Originator and Its Various Biosimilar Drugs? [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/what-is-the-carbon-footprint-of-adalimumab-originator-and-its-various-biosimilar-drugs/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-is-the-carbon-footprint-of-adalimumab-originator-and-its-various-biosimilar-drugs/