Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To estimate the relative risk of malignancy between Janus kinase inhibitors (JAKi), tumour necrosis factor-α inhibitors (TNFi) and placebo in individuals with immune-mediated inflammatory diseases.
Methods: Trial results from clinicaltrials.gov, to February 2024, were systematically extracted to identify Phase II/III/IV, placebo-controlled, randomised clinical trials (RCTs) and long-term extension (LTE) studies of JAKi and TNFi, in adults with rheumatoid arthritis (RA), psoriasis (PsO), psoriatic arthritis (PsA), axial spondyloarthritis (axSpA) and inflammatory bowel disease (IBD). Malignancy was defined using MedDRA version 26.1 Malignant Tumours SMQ. The primary outcome was total cancers excluding non-melanoma skin cancer (NMSC). Secondary outcomes included NMSC, haematological malignancies, and total cancers including NMSC. Bayesian network meta-analyses were performed to estimate Poisson likelihood log rate ratios (logRRs) with 95% credibility intervals (CrI) for malignancy between therapeutic drug classes. Posterior rank probabilities were calculated for each class.
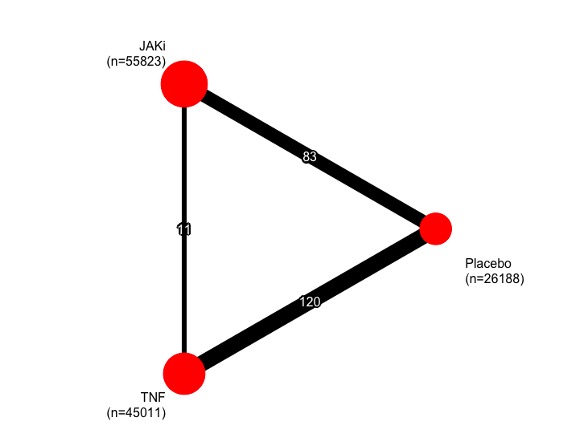
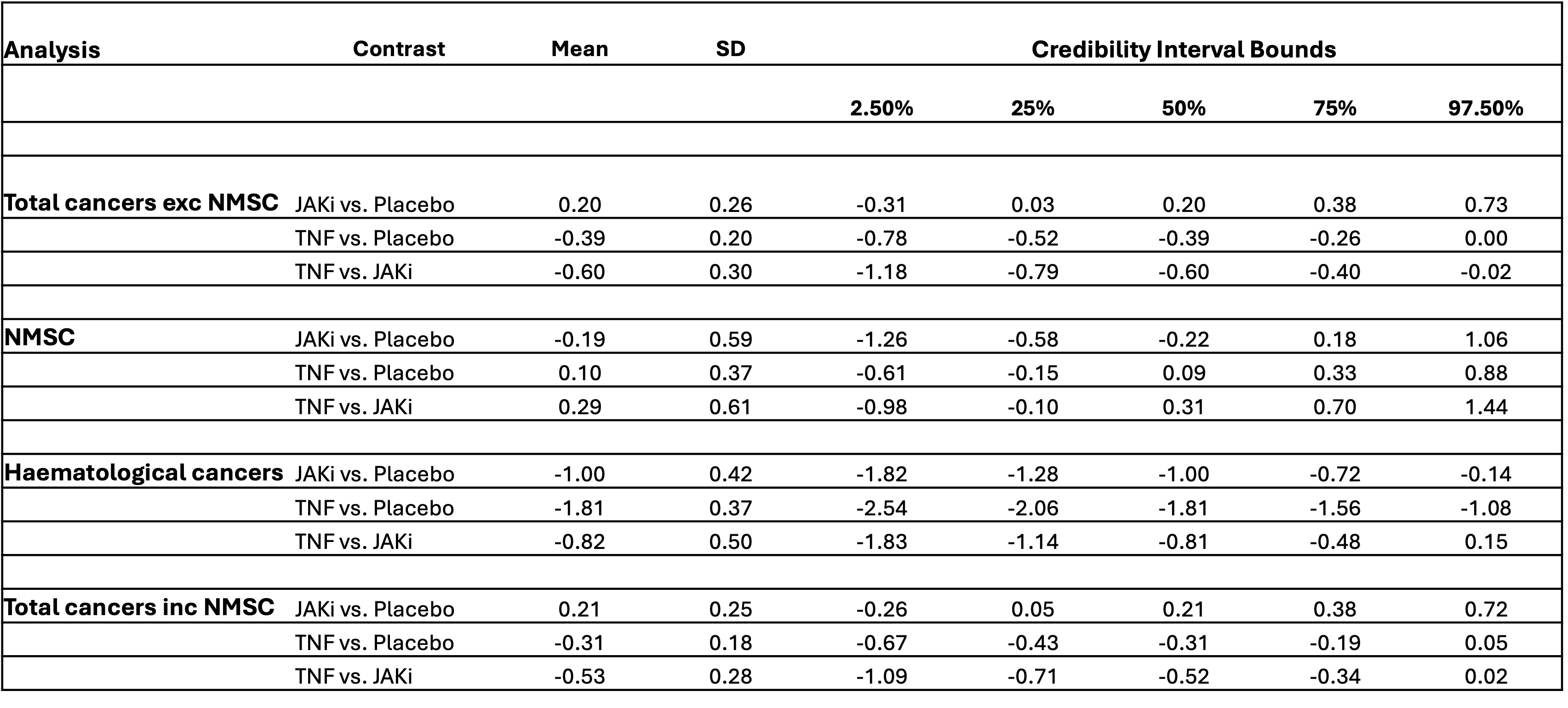
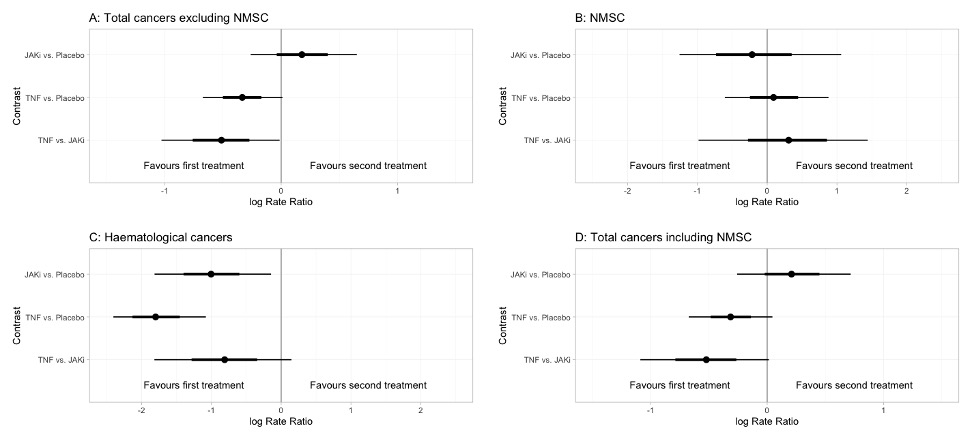
Results: 196 eligible studies were included in our network (Figure 1) totalling 123,773 patients and 133,078 person-years exposure (PYE). There were 68,634 PYE to JAKi, 56,263 PYE to TNFi, and 10,138 PYE to placebo. In total, 1,042 malignancies excluding NMSC were observed. The overall malignancy incidence rate was 7.72 per 1,000 person-years exposure (RA: 8.11, IBD 7.19, PsO 8.92, PsA 7.03, axSpA 2.77). In network meta-analysis of all malignancies excluding NMSCs (Figure 2), the logRR for TNF vs JAKi was -0.60 (95% CrI -1.18 to -0.02). logRRs, relative to placebo, were: TNF -0.31 (95% CrI -0.68 to 0.05); JAKi 0.20 (95% CrI -0.31 to 0.73). Results for NMSC, haematological cancers and total cancers including NMSC are shown in Figure 2. Forest plots of the relative effects for each contrast are shown in Figure 3. Surface under the cumulative ranking curve (SUCRA) scores for the primary outcome of total malignancy excluding NMSC were as follows: TNF 0.97, Placebo 0.41, JAKi 0.12. Analysis of individual JAK inhibitors revealed that Filgotinib was associated with the lowest malignancy risk (SUCRA 0.80) and Baricitinib was associated with the highest (SUCRA 0.05).
Conclusion: TNFi was associated with a significantly lower risk of malignancy excluding NMSC when compared with both JAKi and placebo. The risk of malignancy was similar between JAKi and placebo. Explanations for this pattern could include a protective effect of TNFi or reflect methodological issues such as patient selection bias. Malignancy risk may differ for individual drugs within a treatment class or for different cancer subtypes.
To cite this abstract in AMA style:
Gibson M, Zuckerman B, Adas M, Russell M, Bechman K, Galloway J. Malignancy Risk Between JAK Inhibitors and Anti-TNF Therapy Across Disease Indications: A Bayesian Network Meta-analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/malignancy-risk-between-jak-inhibitors-and-anti-tnf-therapy-across-disease-indications-a-bayesian-network-meta-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/malignancy-risk-between-jak-inhibitors-and-anti-tnf-therapy-across-disease-indications-a-bayesian-network-meta-analysis/