Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Knee osteoarthritis (OA) leads to progressive disability, but approved pharmacologic treatments target analgesia without affecting disease course. Colchicine is well-tolerated and inhibits inflammation thought to modulate OA, but trials to date have yielded mixed results. We tested whether daily colchicine improves pain, function and synovial effusion size in patients with knee OA.
Methods: 120 participants with painful knee OA and radiographic Kellgren-Lawrence grades 2 or 3 were enrolled, excluding individuals with inflammatory or crystal arthritis. Participants were randomized to receive daily colchicine or placebo (1:1) for 12 weeks, while avoiding other anti-inflammatory agents. The primary outcome was change in visual analog scale (VAS) for signal knee pain. Secondary outcomes included changes in the Knee Osteoarthritis Outcome Score (KOOS) subscales (pain, other symptoms, activities of daily living, sports and recreation, quality of life) and in the size of sonographically-identified effusions.
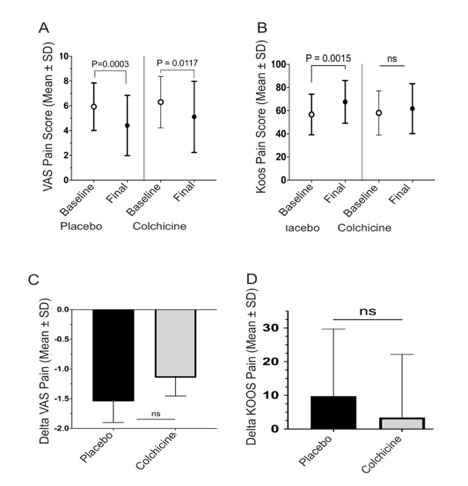
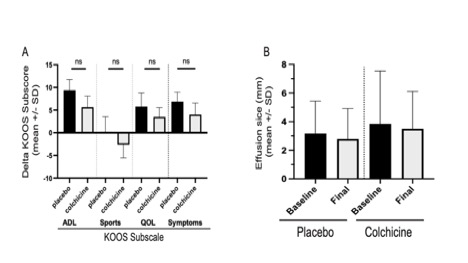
Results: The colchicine and placebo groups were similar at baseline in age, race, gender, ethnicity, VAS pain, and mean levels of serum urate, with a slightly higher mean C-reactive protein (CRP) and radiographic severity in the colchicine group. From baseline to end of treatment for the colchicine vs. placebo groups, we observed no significant between-group differences in mean changes of VAS pain ( -1.1±2.3 vs. -1.5±2.6, p=0.40, Fig. 1C), KOOS subscores (for pain: 3.4±18.7 vs. 9.8±19.9, p= 0.09, Fig. 1D with other KOOS subscores in Fig. 2A) or sonographic size of synovial effusions in millimeters (-0.35 ±3.7 vs. -0.26 ±3.2, p= 0.93, Fig. 2B). These findings for the entire cohort remained when analyzing the per-protocol study completer subset (n=100). The subsets of patients with greater baseline inflammation (CRP or serum urate), or more severe VAS pain or radiographic KL severity, also failed to demonstrate a significant benefit from colchicine vs placebo. Acetaminophen use was permitted as needed, but was not taken less often by patients in the colchicine group.
Conclusion: In this largest double-blind controlled study of colchicine for treatment of knee OA, colchicine failed to improve knee pain, function, or size of synovial effusions. Whether longer treatment with colchicine, higher doses, or a larger cohort would improve pain and function or modify radiographic progression remains to be determined. Current analyses of biomarker levels from these patients’ blood and synovial fluid samples at baseline and 12 weeks will help determine if there is a biochemical effect of colchicine on OA, even if it is not manifesting clinically in a short period of time.
Supported by an Investigator Award from the Rheumatology Research Foundation (to SK-S) and by an investigator-initiated grant from Hikma Pharmaceuticals (to MHP)
To cite this abstract in AMA style:
Samuels J, Tse K, David W, Coronel m, Bomfim F, La Rocca Vieira R, Leung N, Krasnokutsky Samuels S, Toprover M, Pillinger M. CoLchicine for Treatment of OsteoArthritis of the Knee: Clinical Outcomes from a 90-day Double-Blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/colchicine-for-treatment-of-osteoarthritis-of-the-knee-clinical-outcomes-from-a-90-day-double-blind-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/colchicine-for-treatment-of-osteoarthritis-of-the-knee-clinical-outcomes-from-a-90-day-double-blind-placebo-controlled-study/