Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Biosimilar and biologic (b) DMARDs have revolutionized rheumatic disease management in the recent decades. As these drugs become introduced, it is important to understand how they impact pregnancies. We conducted a scoping review to comprehensively synthesize current evidence on biosimilar and non-TNF inhibitor (non-TNFi) bDMARD impacts on perinatal outcomes.
Methods: We conducted a scoping review following the Arksey and O’Malley framework (1). Our biomedical librarian searched Embase, MEDLINE and CENTRAL databases in November 2023. Inclusion criteria were observational studies examining maternal exposure to biosimilars and/or non-TNFi bDMARDs during pregnancy and/or paternal exposure prior to conception in individuals with autoimmune disease. We excluded studies of targeted synthetic DMARDs. We extracted available data on study design, conditions and outcomes, which spanned neonatal, maternal, as well as outcomes during pregnancy and at delivery.
Results: Of 6,712 studies screened, we included 137 studies (62 case reports, 47 cross-sectional, 28 cohort studies) with 77% published between 2018 to 2023. Conditions include Crohn’s disease (16%), ulcerative colitis (14%) and RA (10%). Overall, ustekinumab, vedolizumab and tocilizumab were the most studied drugs (Figure 1).
Among cross-sectional studies, the top non-TNFi bDMARDs studied were ustekinumab and vedolizumab. Biosimilars studied were those for adalimumab, etanercept and infliximab. Of these, neonatal outcomes included small for gestational age (n = 6), low birth weight (n = 17), and neonatal infection (n = 5). Maternal outcomes included preeclampsia (n = 5), disease activity monitored during pregnancy (n = 17), and gestational diabetes (n = 4). Pregnancy outcomes included miscarriage (n = 27), placental abruption (n = 1), and intrauterine growth restriction (n = 5). Lastly, 24 studies reported delivery outcomes (15 on caesarean section, 9 on vaginal delivery).
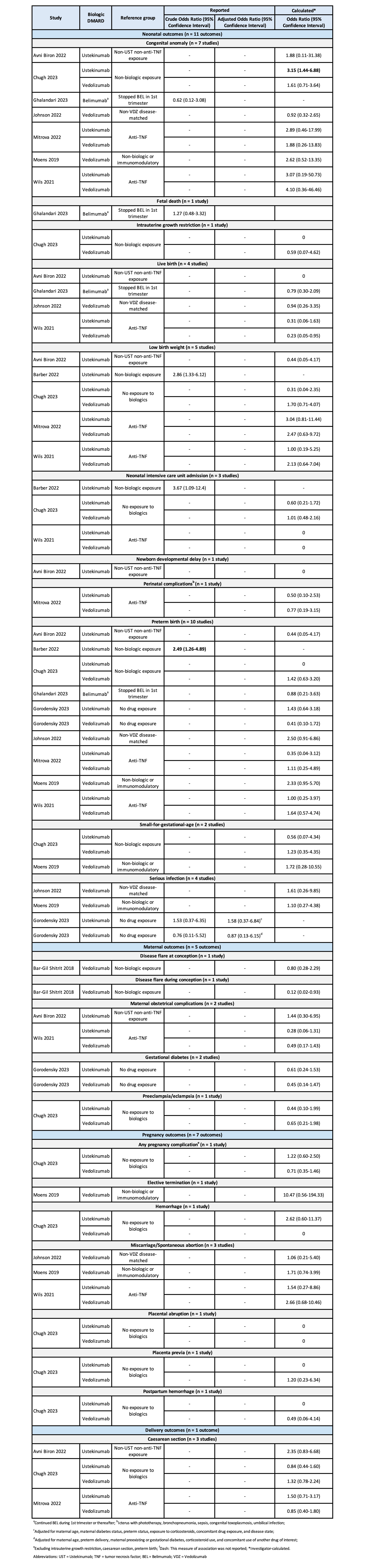
Among 11 cohort studies that reported drug-specific measures of association, we identified significant associations for ustekinumab and congenital anomaly (OR 3.15 [95% confidence interval (CI) 1.44-6.88]) and preterm birth (OR 2.49 [95% CI 1.26-4.89]) (Table 1). Maternal outcomes of preeclampsia were reported for ustekinumab (OR 0.44 [95% CI 0.10-1.99]) and vedolizumab (OR 0.65 [95% CI 0.21-1.98]), as well as disease flare at conception for vedolizumab (OR 0.80 [95% CI 0.28-2.29]). Pregnancy outcomes such as miscarriage were reported for ustekinumab (OR 1.54 [95% CI 0.27-8.86]) and vedolizumab (ORs ranged 1.06 to 2.66). The delivery outcome of caesarean section was reported for ustekinumab (ORs ranged 0.84 to 2.35) and vedolizumab (ORs ranged 0.85 to 1.32). No biosimilars were examined.
Conclusion: Our scoping review provides a comprehensive synthesis of evidence to date on the perinatal use of biosimilars and non-TNFi bDMARDs, suggesting small sample sizes, limited maternal outcomes and no paternal outcomes. Findings highlight gaps in literature where evidence is needed for providers and patients to make informed treatment decisions that minimize maternal-fetal risk.
(1) Arksey, H., O’Malley, L. Scoping studies: towards a methodological framework. Volume 8, 2008.
To cite this abstract in AMA style:
Cheng V, Amiri N, Cheng V, Ellis U, Cragg J, Harrison M, De Vera M. Pregnancy Outcomes of Biosimilars and Non-TNF Inhibitor Biologic Disease-Modifying Antirheumatic Drugs: A Scoping Review [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pregnancy-outcomes-of-biosimilars-and-non-tnf-inhibitor-biologic-disease-modifying-antirheumatic-drugs-a-scoping-review/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregnancy-outcomes-of-biosimilars-and-non-tnf-inhibitor-biologic-disease-modifying-antirheumatic-drugs-a-scoping-review/