Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Early recognition and treatment of temporomandibular joint (TMJ) arthritis in children with juvenile idiopathic arthritis (JIA) is of high importance given its impact on quality of life as its use is required for daily functions such as talking and eating. Untreated TMJ inflammation in children can lead to growth abnormalities leading to permanent facial deformities. Little is known about the effectiveness of different types of systemic therapies for the treatment of TMJ arthritis as no prospective studies have been performed in the biologic era. The current study is a secondary data analysis of the open label portion of the JUNIPERA trial, a prospective study evaluating the efficacy of secukinumab in patients with juvenile enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA). The objective is to evaluate the efficacy of secukinumab in treating TMJ involvement in children with ERA and JPsA.
Methods: Biologic naïve patients with ERA (age 4 – 17 years) and JPsA (age 2 – 17 years) enrolled in the JUNIPERA trial began subcutaneous secukinumab (75/150 mg in patients < 50/≥50 kg). All patients received doses at week 0, 1, 2, 3, 4, 8, and 12 as part of the open label portion of the trial. Disease assessment measures including 27-joint Juvenile Arthritis Disease Activity Score (JADAS-27), physician global assessment, and patient global assessment were recorded at each visit. TMJ involvement was defined as having joint tenderness, joint swelling, or limitation of motion. A repeated measure logistic regression (RMLR) was used to assess proportion of patients with TMJ involvement at each timepoint through week 12.
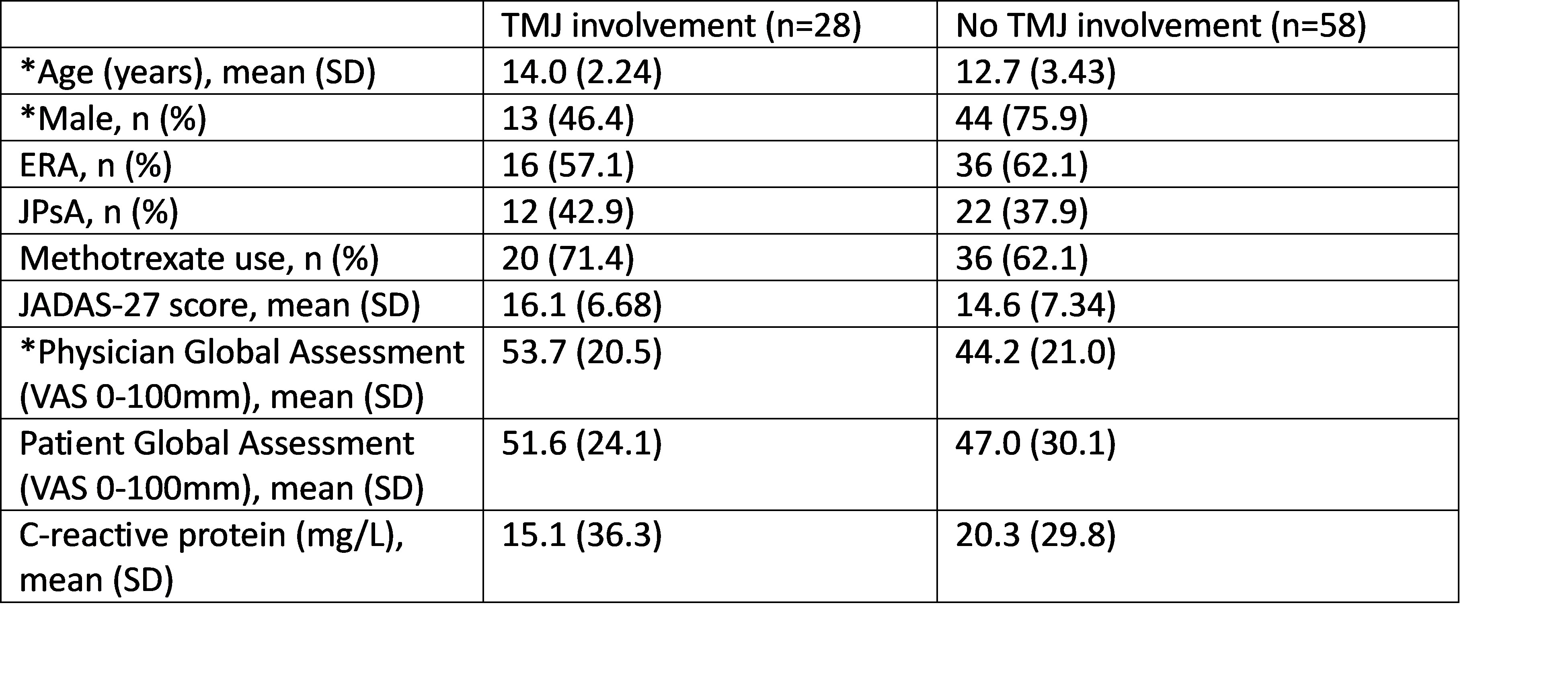
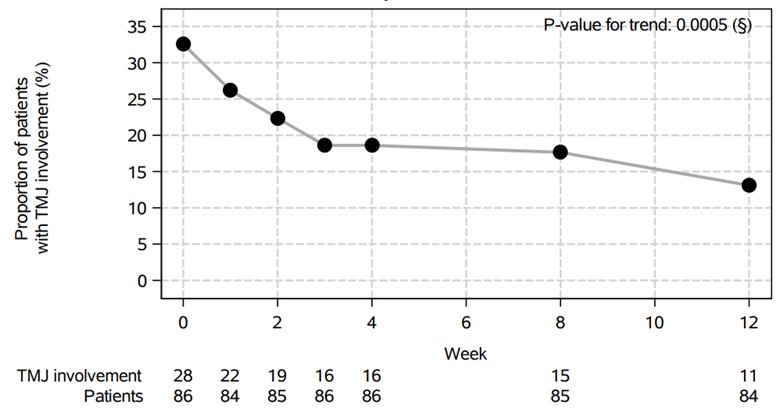
Results: A total of 86 patients with JIA (52 with ERA and 34 with JPsA) were enrolled and 28 patients had TMJ involvement at week 0 (32.6%). All but two patients had TMJ assessments up to week 12 on secukinumab. Baseline demographics and disease activity measures in patients with and without TMJ involvement are summarized in Table 1. Patients with TMJ involvement were more likely to be female and were older than those without. The physician global assessment trended towards (p=0.05) having a higher score in patients with TMJ involvement compared to those without. The two groups had no statistically significant differences in JADAS-27 or patient global assessment. The effectiveness of secukinumab on TMJ involvement is shown in Figure 1. RMLR demonstrated a statistically significant trend in reduced proportions of patients with TMJ involvement through week 12 (p=0.0005).
Conclusion: Studies on the treatment of TMJ arthritis are limited given the challenges in differentiating TMJ arthritis from dysfunction without use of imaging. The current post-hoc analysis demonstrates secukinumab improved signs and symptoms of TMJ involvement in patients with ERA and JPsA. Additional prospective studies are needed to compare the efficacy of systemic therapies in treating TMJ arthritis to reduce permanent damage from undertreated TMJ arthritis using imaging as an endpoint.
To cite this abstract in AMA style:
Kerski M, Mohan S, Vizcaya C, Sutariya R, Bao W, Stoll M. Effectiveness of Secukinumab in TMJ Symptoms in Children with JPsA and ERA: A Secondary Data Analysis of JUNIPERA [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-secukinumab-in-tmj-symptoms-in-children-with-jpsa-and-era-a-secondary-data-analysis-of-junipera/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-secukinumab-in-tmj-symptoms-in-children-with-jpsa-and-era-a-secondary-data-analysis-of-junipera/