Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The FiRst Line Options for sJIA Treatment (FROST) trial was a prospective observational study designed to compare the effectiveness of 4 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Consensus Treatment Plans (CTPs) in new-onset systemic JIA (sJIA). The primary study showed that initial biologic therapy was the most common treatment approach (86% of patients). 30/53 (57%) of patients achieved a state of Clinically Inactive Disease (CID) without concurrent glucocorticoid (GC) use at 9 months. The current study aimed to evaluate clinical outcomes and safety of the FROST cohort at 2- and 3-years after baseline.
Methods: sJIA patients enrolled in FROST continued follow up visits every 6 months (+/- 3 month window) in the CARRA Registry beyond the initial study. All medications changes were made by the treating physician and patient/family. Adverse Events were recorded, along with prespecified safety events, including Macrophage Activation Syndrome (MAS). The primary study outcome was CID (Wallace) without use of GC at 2- and 3-years. Selected secondary outcomes included CID irrespective of current GC use, cJADAS-10 ≤2.5 without current fever or GC use, GC use independent of CID, and proportion of the entire cohort achieving CID at any point during follow up.
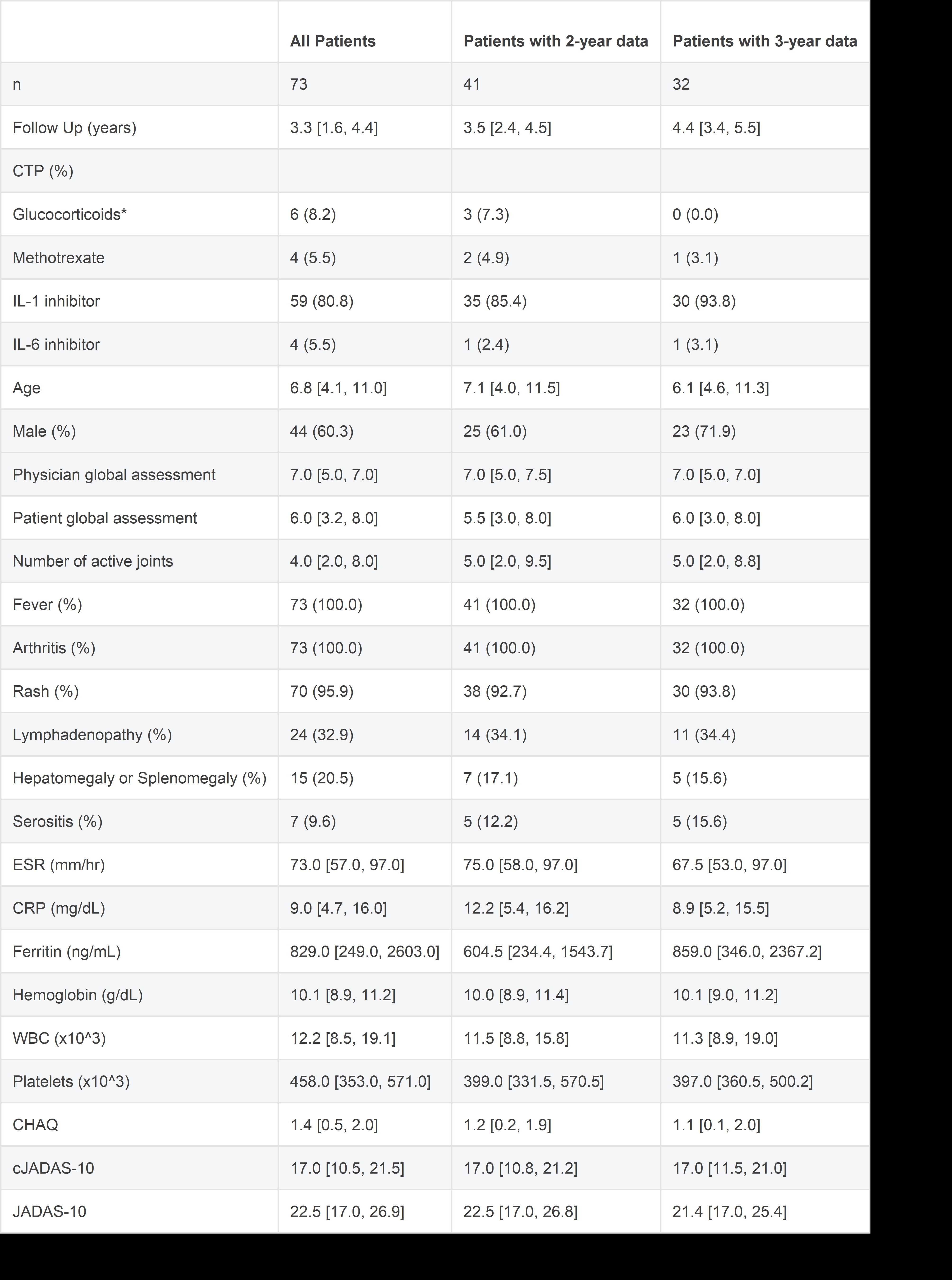
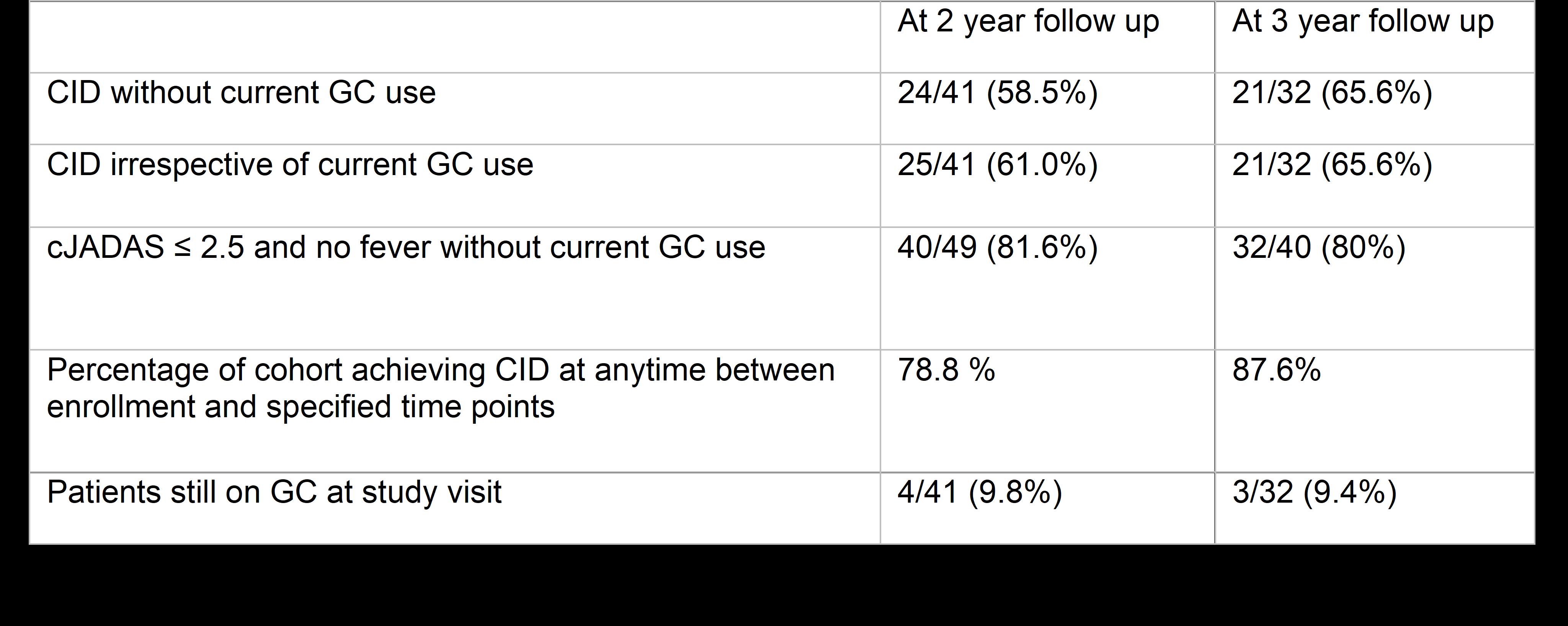
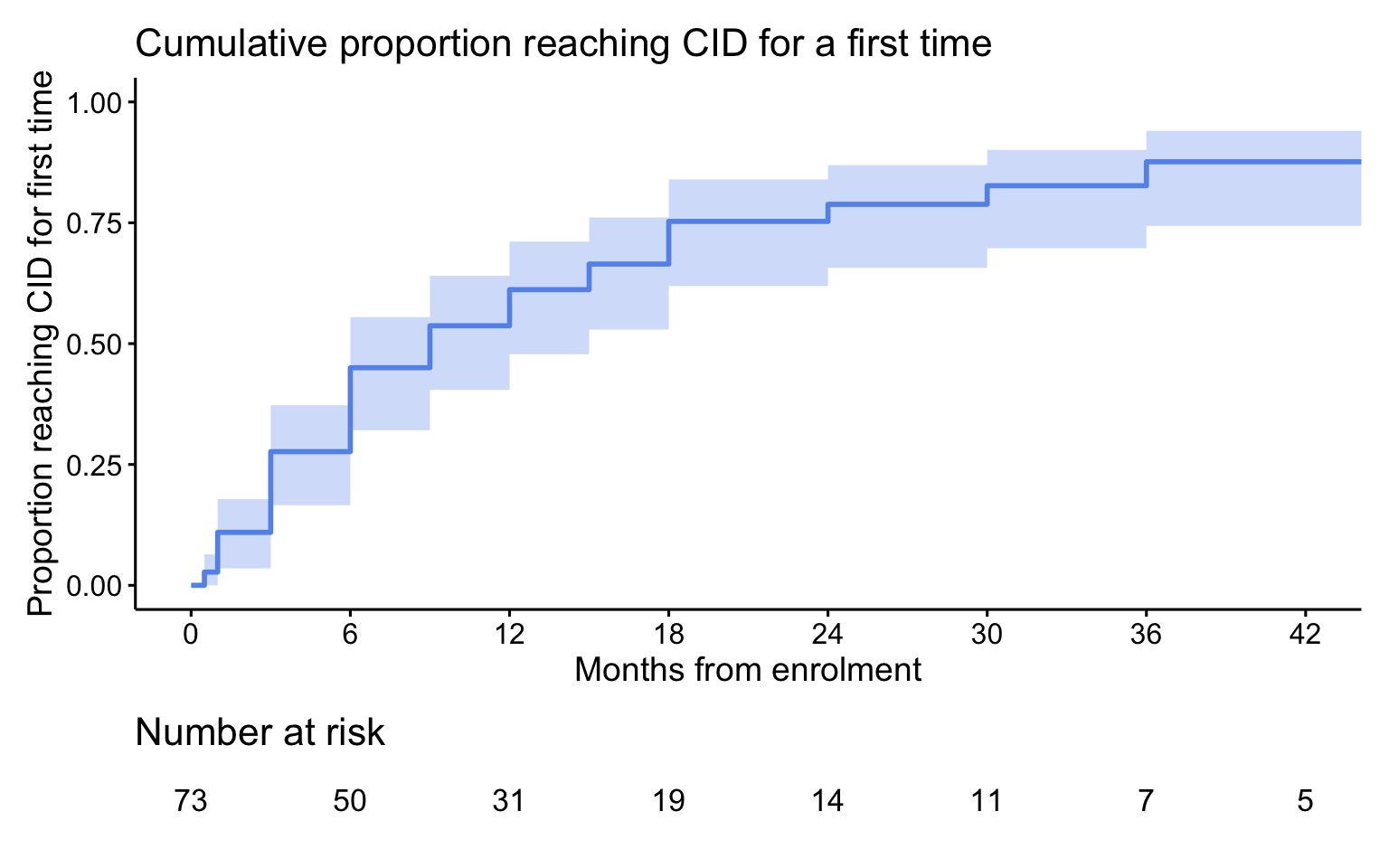
Results: Of the 73 subjects enrolled in FROST, 50 had a 2-year visit and 39 had a 3-year visit. Due to missing data, CID and/or cJADAS could not be calculated for all subjects reducing the number included in the analysis. For the primary outcome of CID without concurrent GC use there were 41 patients at 2 years and 32 patients at 3 years with complete data. Biologic CTPs using an IL-1 or IL-6 inhibitor were the most common initial treatment for subjects with follow up at 24 months (87.8%) and 36 months (96.9%). 58.5% (24/41) of patients at 2-years, and 65.6% (21/32) at 3-years were in CID without concurrent GC use. The cumulative incidence of achieving CID at any time reached 87.6% by 3-years. Almost all patients were off GC at 2-years (90.5%) and at 3-years (90.6%). Twenty-one subjects (28.7%) were off all medications at the time of their most recent study visit. There were 29 adverse events (AEs) reported in 19 subjects, including 15 Serious AEs (SAEs) in 10 subjects during follow up. The most frequent SAEs were sJIA flare and Macrophage Activation Syndrome (MAS). Two SAEs were infections requiring hospitalization. Overall frequency of any AE was 12.7 per 100-patient years and for SAEs was 6.6 per 100-patient years. There was 1 death due to acute liver failure occurring 2.6 years after enrollment. No patients were reported to have developed sJIA lung disease.
Conclusion: Patients with sJIA enrolled in FROST continued to improve during the 2nd and 3rd year of follow-up with the majority of patients followed achieving CID and able to discontinue GCs. SAEs were not common and predominantly were due to flare or MAS. The FROST cohort will continue to be followed in the CARRA Registry, providing further longer-term information about the current course of sJIA patients.
Disclosures: T. Hahn: None; G. Tomlinson: None; Y. Kimura: None; V. Del Gaizo: None; C. Valdes: Genentech, 3; T. Beukelman: UCB, 1.
To cite this abstract in AMA style:
Hahn T, Tomlinson G, Kimura Y, Del Gaizo V, Valdes C, Beukelman T. Two- and 3-Year Outcomes of the Childhood Arthritis and Rheumatology Research Alliance FROST Study of New-onset Systemic JIA Treatment [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/two-and-3-year-outcomes-of-the-childhood-arthritis-and-rheumatology-research-alliance-frost-study-of-new-onset-systemic-jia-treatment/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-and-3-year-outcomes-of-the-childhood-arthritis-and-rheumatology-research-alliance-frost-study-of-new-onset-systemic-jia-treatment/