Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: TNF-alpha inhibitors are widely used in the field of rheumatology and have been linked to various possible adverse events, including demyelinating diseases. This association has not been proven, and the general practice is to avoid these medications in patients with demyelinating diseases or multiple sclerosis. Prior reported cases of demyelinating disease mainly involve patients receiving etanercept. Reported findings include nonspecific neurologic symptoms such as confusion, ataxia, paresthesia, facial nerve palsy, optic neuritis, hemiparesis, transverse myelitis, and Guillain-Barré syndrome. These symptoms partially or completely improved after stopping the medication. The estimated incidence of demyelinating disease in patients receiving etanercept was 31 per 100,000 patient-years of exposure compared to 4-6 per 100,000 in the general population.
We aim to evaluate the risk of developing demyelinating disease in patients receiving different TNF-alpha inhibitors. We used Trinetx database which encompasses data of more than120 million patients from 5 countries.
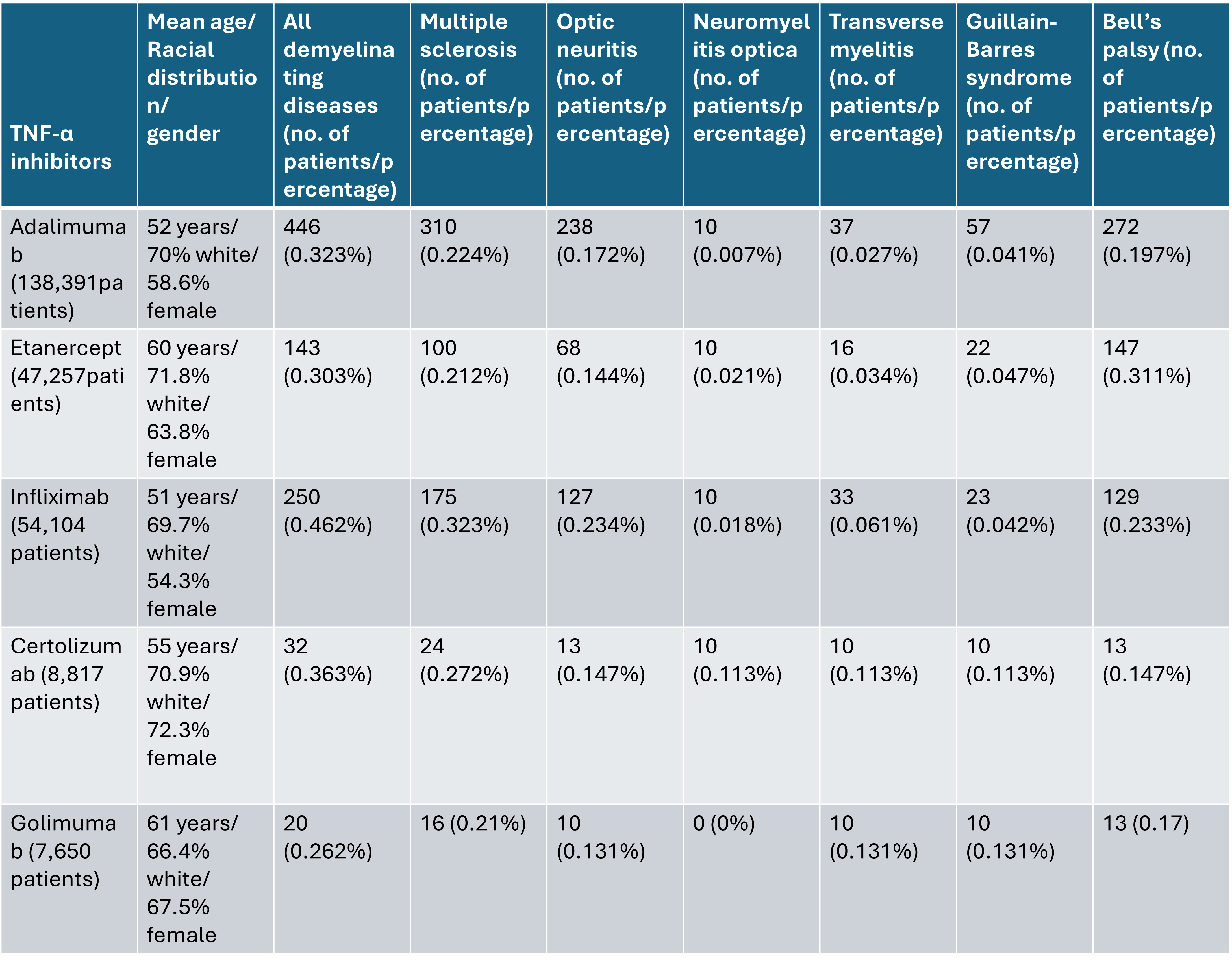
Methods: Using the Trinetx database, we identified five different cohorts for each of the TNF inhibitors, excluding patients on other TNF inhibitors (adalimumab, etanercept, infliximab, certolizumab pegol, and golimumab). The index event was the first time the medication appeared in the patient’s chart. We studied outcomes of (demyelinating diseases of any type, Multiple sclerosis, optic neuritis, neuromyelitis optica, transverse myelitis, Guillain-Barré syndrome, and Bell’s palsy) occurring within 10 years of index event. Patients with these outcomes before starting TNF-alpha inhibitors were excluded. We then compared the risk of developing demyelinating diseases in patients receiving etanercept versus adalimumab, and infliximab versus adalimumab, excluding patients with the outcome before starting medication.
Results: Overall, patients on different TNF inhibitors had a comparable risk of developing demyelinating disease, with an increased risk associated with infliximab. (table 1)
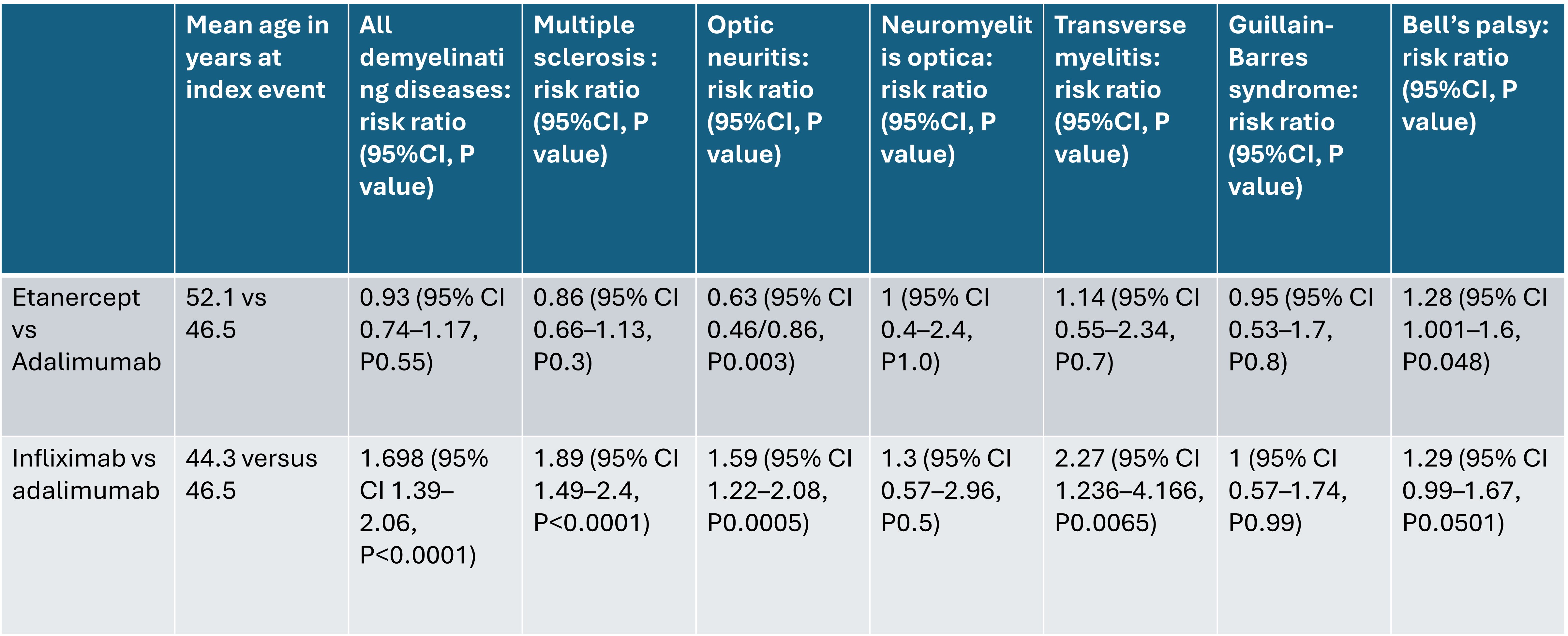
When we compared etanercept versus adalimumab, there was no statistically significant difference between the two medications, apart from a slightly lower risk of optic neuritis with etanercept. However, comparing infliximab versus adalimumab, infliximab had a statistically significant increased risk of developing demyelinating disease, multiple sclerosis, optic neuritis, and transverse myelitis. (table 2)
Conclusion: Compared to prior reports of a higher risk of developing demyelinating disease with etanercept, our study showed that patients on etanercept do not have an increased risk of demyelinating disease compared to other TNF inhibitors, while infliximab has a statistically significant higher risk compared to adalimumab.
To cite this abstract in AMA style:
Ali R, Gonzalez Moret Y, Hussein R, Rodriguez F. Comparative Risk of Demyelinating Diseases Among Patients on TNF-Alpha Inhibitors: A Cohort Study Using the TriNetX Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparative-risk-of-demyelinating-diseases-among-patients-on-tnf-alpha-inhibitors-a-cohort-study-using-the-trinetx-database/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-risk-of-demyelinating-diseases-among-patients-on-tnf-alpha-inhibitors-a-cohort-study-using-the-trinetx-database/