Session Information
Date: Saturday, November 16, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The growing number of randomized clinical trials (RCTs) in idiopathic inflammatory myopathies (IIMs) points to a promising future for therapeutics in IIM. Adequate representation of race/ethnicity and equitable geographic distribution of enrolling sites are crucial for the generalizability of RCT results. Currently, little is known about demographic diversity in IIM RCTs. In this systematic literature review, Myositis International Health & Research Collaborative Alliance (MIHRA) Global Equity and Clinical Trial Readiness Cores aimed to summarize the current state of racial, ethnic, sex, and geographical representation in IIM RCTs to inform the future steps for equitable representation in IIM RCTs.
Methods: We performed a systematic literature review using PubMed, EMBASE, CINAHL Complete, Europe PMC, ClinicalTrials.gov, and the International Clinical Trials Registry Platform. All pharmacologic phase 1, 2, 2b/3, and 3 IIM RCTs published between 2010-2023 were included. Two authors independently reviewed the titles, abstracts, and full texts and extracted the data on demographics, year of publication, phase of trial, sponsor category (academic, government, industry), and location of enrolling sites (continent, country, ZIP+4 for U.S. sites). U.S. enrollment sites were categorized at the county level using Urban-Rural Classification Scheme (small, medium, large metropolitan, and non-metropolitan) and into Medically Underserved Areas (yes/no) according to the U.S. Department of Health & Human Services data. Diverse populations were defined as enrolling ≥20% non-White and/or ≥45% female participants based on U.S. Census Data.
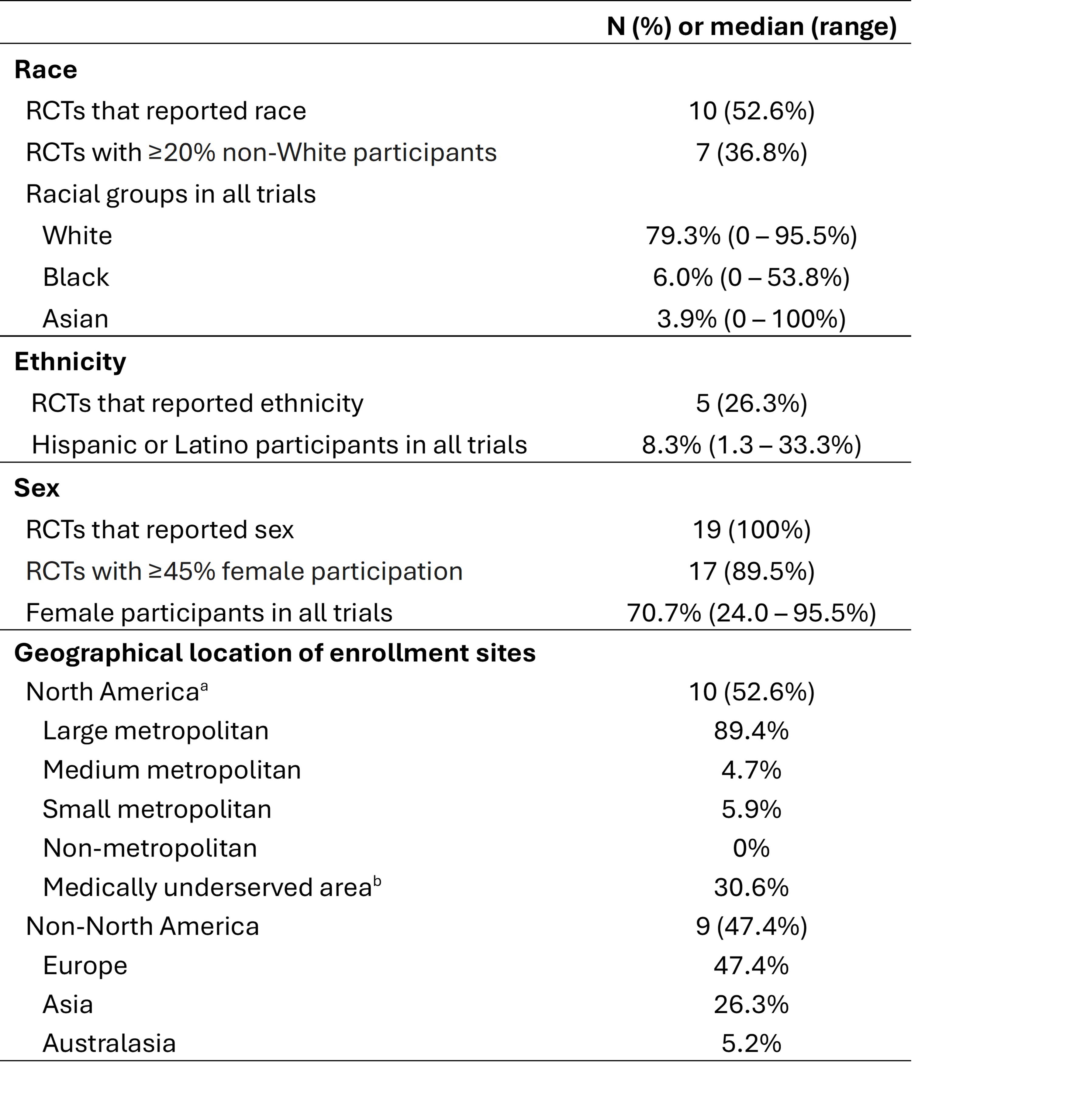
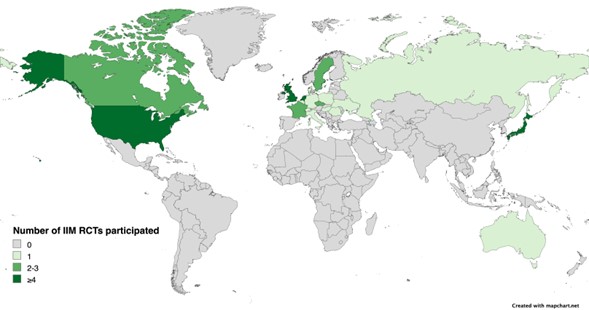
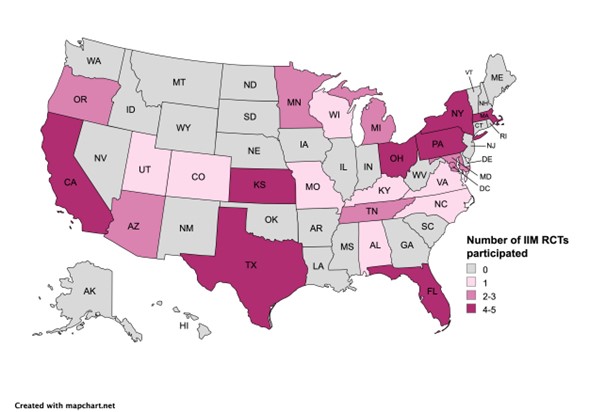
Results: A total of 19 RCTs with a total of 1221 participants (mean age 63.7 years) fulfilled the inclusion/exclusion criteria of this SLR. Race of the participants was reported in 10 (52.6%) RCTs, with 4 (21.1%) only reporting the number of White participants (Table 1). Black and Asian participants represented 6.2% and 2.6% of the trial cohort, respectively (the latter excluded studies conducted exclusively in Asia). Ethnicity was reported only in 5 (26.3%) RCTs, and 2 (10.5%) others reported race as ethnicity. Hispanic or Latino participants represented 6.7% of the trial cohort. Sex was reported in all studies, with a female-to-male proportion of 1.4 in IBM trials, and 2.6 in non-IBM trials. Most trials (n=17, 89.5%) had over 45% female participants. The majority of the RCTs were multicentric (73.7%) and most commonly conducted in North America (n=10) followed by Europe (n=9), Asia (n=5), and Australasia (n=1) (Figure 1). There was a total of 85 U.S. enrolling sites, from which 76 (89.4%) were located in large metropolitan areas, and 26 (30.6%) in Medically Underserved Areas (Figure 2).
Conclusion: The reporting of race and ethnicity in IIM RCTs is limited. The distribution of RCT enrollment sites is confined to North America, Europe, and Japan with no sites in South America or Africa. Given the dearth of knowledge of IIM phenotype, outcome, and treatment response in several areas of the world, collaborative efforts between researchers and sponsors are required to develop infrastructure to secure increased access, recruitment and retention of diverse populations in IIM RCTs.
bBased on Health the Resources and Services Administration designation of MUA lacking access to primary care services at the county level (last updated 2023).
To cite this abstract in AMA style:
Silva R, Carpio Tumba M, Gupta S, Gupta L, Machado P, Paik J, Louden D, Saketkoo L, Sattui S, Saygin D. Racial, Ethnic, Sex, and Geographical Diversity in Myositis Clinical Trials: Future Steps for Equitable Representation by the MIHRA Global Equity and Clinical Trial Readiness Cores [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/racial-ethnic-sex-and-geographical-diversity-in-myositis-clinical-trials-future-steps-for-equitable-representation-by-the-mihra-global-equity-and-clinical-trial-readiness-cores/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/racial-ethnic-sex-and-geographical-diversity-in-myositis-clinical-trials-future-steps-for-equitable-representation-by-the-mihra-global-equity-and-clinical-trial-readiness-cores/