Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Immunocompromised patients constitute a high-risk group for severe COVID-19 outcomes and those on B cell depleting therapy (BCTD) are among the most vulnerable. Previous work by our group has documented these risks in the pre-Omicron era as well as the effectiveness of monoclonal antibody therapy in attenuating severe outcomes. Recent work has documented the persistence of this clinical vulnerability among patients on BCTD into the Omicron era. In the Omicron era outpatient management is centered about the use of oral antiviral therapy with nirmatrelvir/ritonavir or molnupiravir. Data are now emerging on their effectiveness against Omicron infections across the broad category of patients classified as high risk by CDC, however their effectiveness in patients on BCDT remains unknown. We conducted an observational cohort study examining the effectiveness of oral nirmatrelvir/ritonavir or molnupiravir on COVID-19 outcomes in patients receiving BCDT for immune mediated inflammatory diseases (IMIDs).
Methods: Data were derived from the EHR for this observational cohort study. Inclusion criteria: adults (≥18 years) with 1) positive COVID-19 test 12/19/21-3/1/24, 2) with IMID ICD-10 code (RA, ANCA vasculitis, SLE, DM, Sjogren’s, CNS inflammatory disease, pemphigus, IgG4-RD, AIHA, GBS) within 3 years prior to positive test, 3) receipt of rituximab or ocrelizumab within 1 year prior to positive test. The primary outcome was death or hospitalization from COVID-19 within 28 days. Data collected were described using counts, means, standard deviations, medians, quartiles and ranges for all continuous variables and counts and percentages for categorical variables. R 4.3.1 software was used for all analyses.
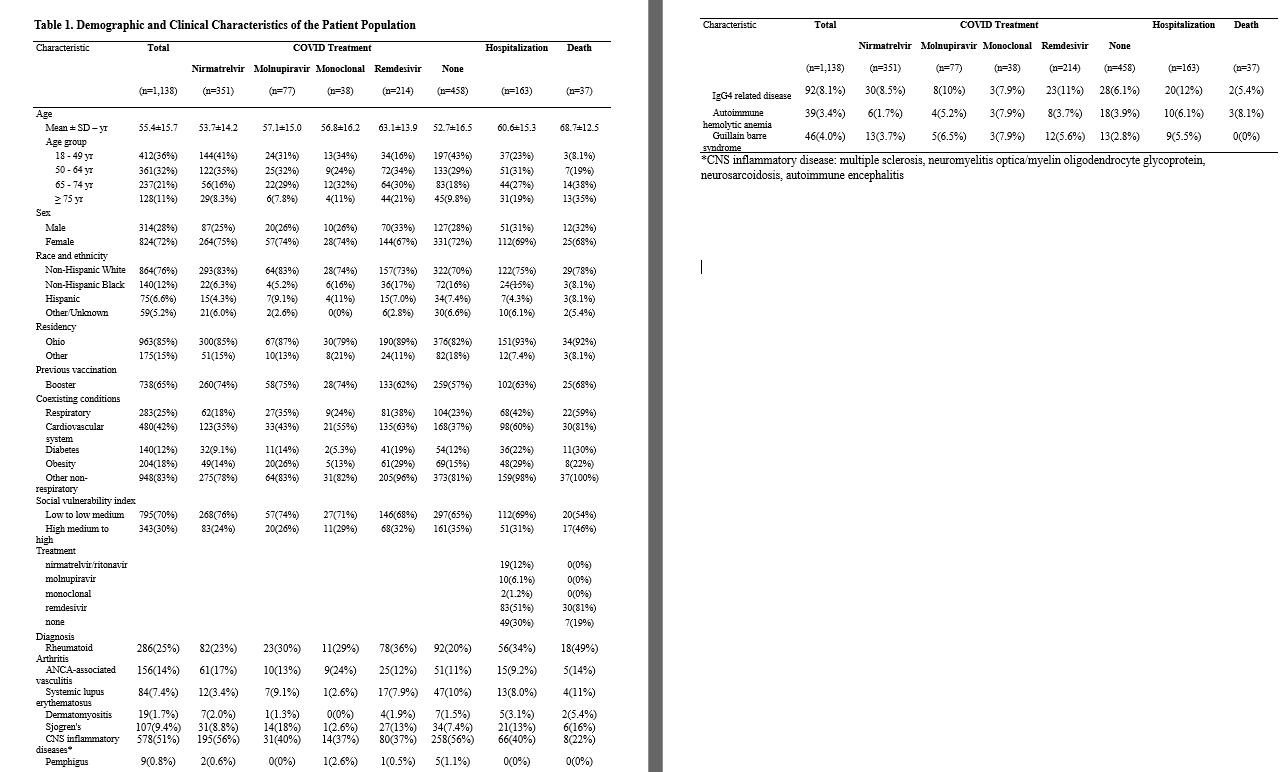
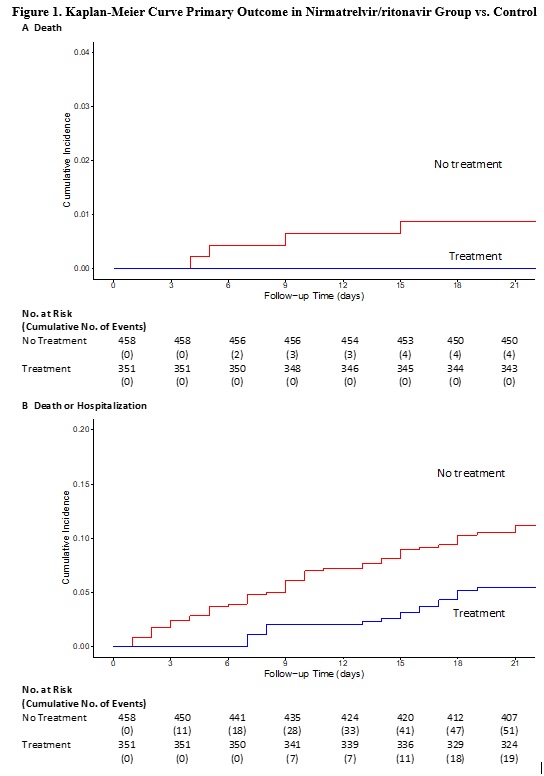
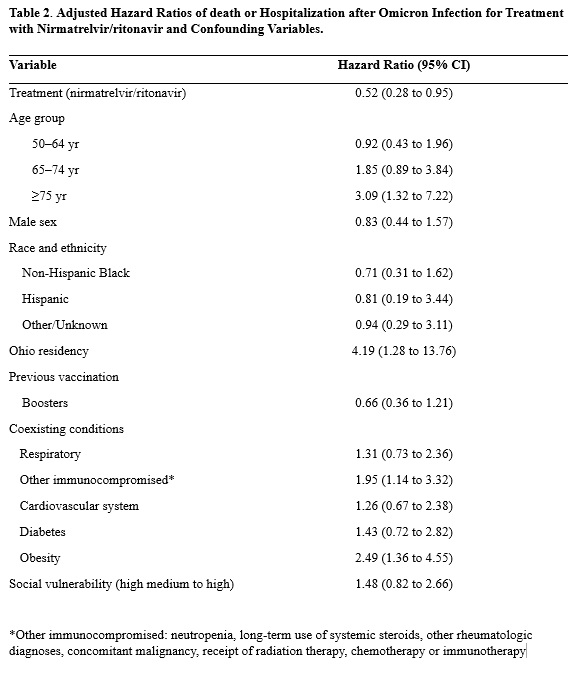
Results: 1,138 patients met inclusion criteria (Table 1). 50.8% (n=578) had a diagnosis code for CNS inflammatory disease, 57.3 % (n=652) had rheumatologic diagnoses and 16.3% (n=186) had other diagnoses. 278 patients had >1 ICD indication for BCDT. 687 received rituximab, 451 ocrelizumab. For outpatient treatment, 30.8% (n=351) received nirmatrelvir/ritonavir, 6.8% (n=77) molnupiravir, 3.3% (38) monoclonals, 18.9% (n=215) remdesivir, and 40.2% (n=458) received no treatment. Of the entire cohort, 14.3% (n= 163) were hospitalized and 3.3% (n=37) died. In the nirmatrelvir/ritonavir group, its use was associated with significantly decreased incidence of hospitalization and death (Figure 1). Using a multivariate Cox-regression model, nirmatrelvir/ritonavir remained associated with lower incidence of primary outcome (aHR 0.52 (0.28 to 0.95) (Table 2). NNT to prevent hospitalization or death for nirmatrelvir/ritonavir was 17. Rate of death or hospitalization was higher for rituximab patients compared to ocrelizumab (17.8% vs. 11.3%).
Conclusion: In this observational cohort study of IMIDs patients with indications for anti-CD20 therapy, treatment with nirmatrelvir/ritonavir was associated with lower rates of hospitalization and death from COVID-19. Despite improved outcomes of SARS-CoV-2 infection during Omicron compared to prior epochs, patients receiving BCDT remain high risk for poor outcomes and should continue to be offered early outpatient treatment with nirmatrelvir/ritonavir.
To cite this abstract in AMA style:
Calabrese C, wang X, Duggal A, Sacha G, Huang S, Calabrese L. Use of Outpatient Antiviral Therapy and Severe Outcomes from SARS-CoV-2 Omicron Infection in Patients with Immune Mediated Diseases on B Cell Depleting Agents [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/use-of-outpatient-antiviral-therapy-and-severe-outcomes-from-sars-cov-2-omicron-infection-in-patients-with-immune-mediated-diseases-on-b-cell-depleting-agents/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-outpatient-antiviral-therapy-and-severe-outcomes-from-sars-cov-2-omicron-infection-in-patients-with-immune-mediated-diseases-on-b-cell-depleting-agents/