Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The ACR recommends SARS-CoV-2 vaccination for all patients with rheumatic diseases, but it is unknown how patients with SLE will respond to the vaccine, given aberrant immune systems and frequent use of immune suppressing medications.
Methods: Using biobanked serum from before and following vaccination, we investigated the immunogenicity of two doses of a SARS-CoV-2 mRNA vaccine in 87 adult SLE patients and 16 adult healthy controls. ELISA was used to assess the amount of ancestral strain (D614G) anti-spike antibodies being produced, reported as area under the curve (AUC); an AUC < 2 was considered to be a minimal response, 2-6 blunted response, and > 6 full response. To determine antibody avidity, 7M urea was used as a chaotropic agent (avidity = [7M urea AUC/PBS AUC] x100), and neutralization was assessed using a lentiviral pseudovirus neutralization assay, reported as ID50 (ID50 of 100+ being considerate adequate).
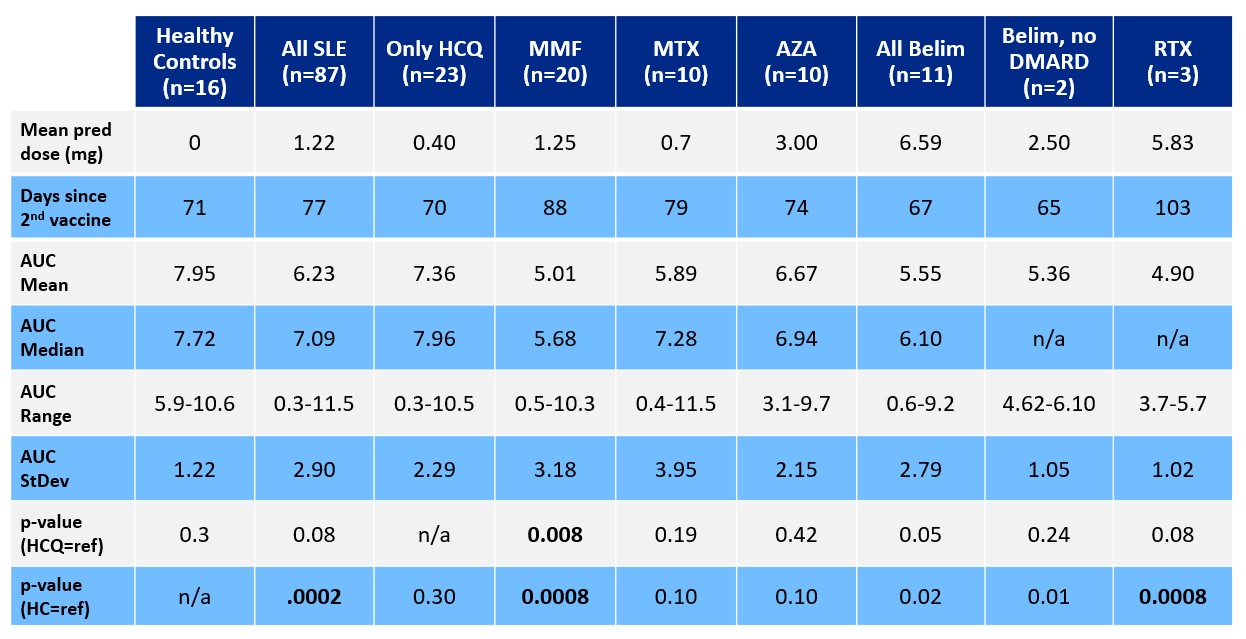
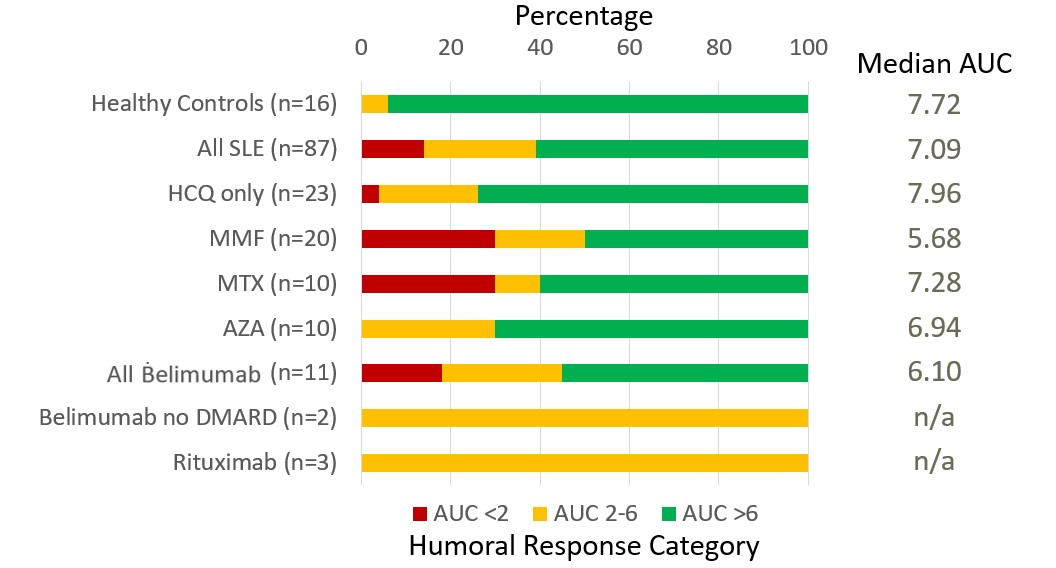
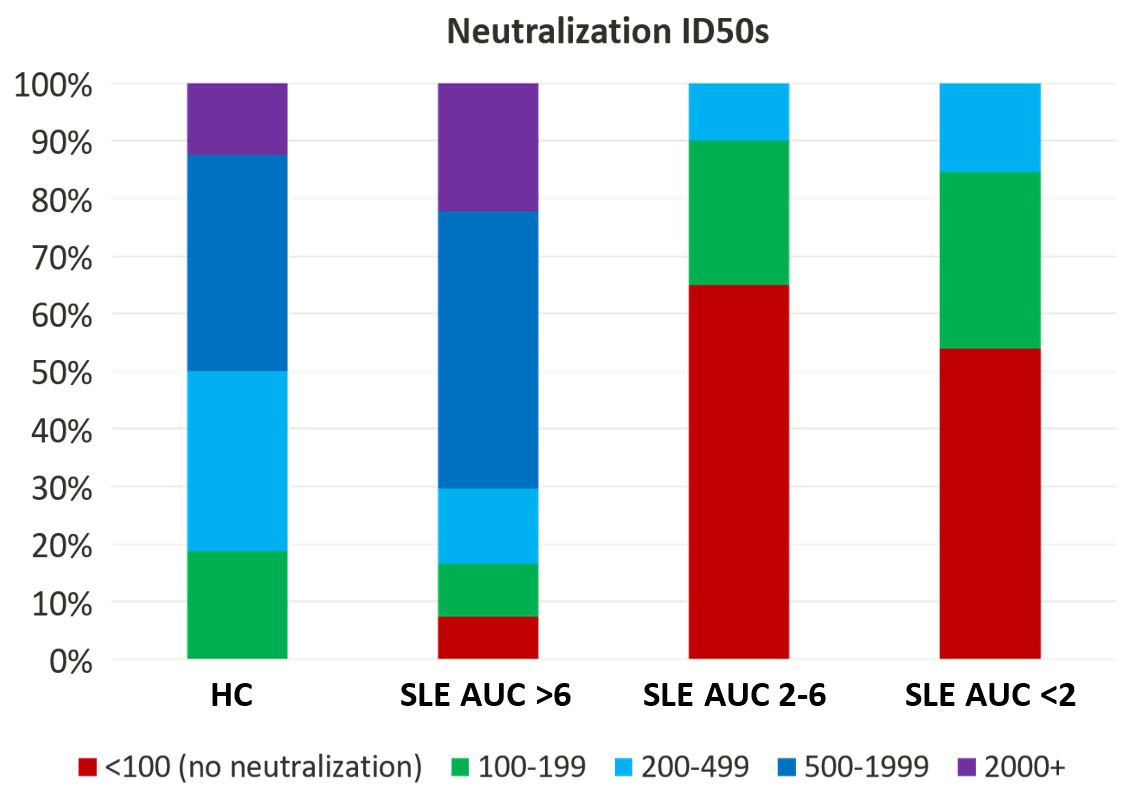
Results: As seen in Table 1, healthy controls had a higher AUC (mean 7.95, median 7.72, range 5.88-10.60) compared to the 87 SLE patients (mean 6.23, median 7.09, range 0.34-11.5; p=0.0002). The responses of 23 SLE patients who were receiving no immunosuppressive medications (mean 7.36, median 7.96, range 0.34-10.5) were not different, as a group, from those of healthy controls (p=0.3). Among SLE patients, neither disease activity nor prednisone dose (0-60 mg; p=0.8) was associated with AUC. Compared to healthy controls (p=0.0008) or SLE patients not taking immunosuppressants (p=0.008), SLE patients treated with rituximab (n=3, mean 4.90, range 3.75-5.70) or mycophenolate (n=20, mean 5.01, median 5.68, range 0.50-10.34) had significantly reduced antibody production. As seen in Figure 1, 94% of healthy controls had a full response, with 1 person (6%) having a blunted response, just below the threshold of 6. Among SLE patients, however, more than one third had a blunted response, and more than 10% had minimal-to-no response. Impaired antibody production was most notable in SLE patients treated with mycophenolate or methotrexate, with 30% of the patients in each group showing minimal-to-no response. Avidity studies revealed no significant difference between healthy control avidity and SLE patient avidity (p=0.61). As seen in Figure 2, despite similar avidity, neutralization differed between healthy controls and SLE patients: whereas 100% of healthy controls neutralized ancestral strain SARS-CoV-2, only 92% of SLE patients who had an AUC > 6 neutralized adequately; intriguingly, neutralization of SARS-CoV-2 was observed in 66% of SLE patients with an AUC 2-6 and in 55% of SLE patients with an AUC < 2.
Conclusion: We show that treatment with either rituximab or mycophenolate, but not prednisone, significantly blunts the humoral immunogenicity of SARS-CoV2 mRNA vaccines. Our results also suggest that, while the anti-spike antibodies produced by SLE patients have similar avidity, they have decreased ability to neutralize virus in comparison to the antibodies produced by healthy controls.
To cite this abstract in AMA style:
Sadun R, Crair D, Walter E, St.Clair E, Pisetsky D, Eudy A, Clowse M, Rogers J, Sun K, Criscione-Schreiber L, Maheswaranathan M, Doss J, Valencia S, Moody M. Anti-Spike Antibodies in SARS-CoV-2-Vaccinated SLE Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anti-spike-antibodies-in-sars-cov-2-vaccinated-sle-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-spike-antibodies-in-sars-cov-2-vaccinated-sle-patients/