Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Biologic response modifiers (BRMs), also known as “biologics,” have become essential tools in treating various chronic inflammatory conditions. However, the immunosuppression caused by these agents increases the risk of opportunistic infections. Coccidioidomycosis (CM), also known as San Joaquin Valley Fever, is primarily found in the soil in specific geographic regions of the southwestern US (e.g., Arizona, California). Though the majority of individuals who are exposed to coccidioidomycosis may only have a mild flu-like illness, it can become life-threatening, especially in immunosuppressed individuals. Before starting biologic therapy, it is a standard practice to conduct testing for tuberculosis and hepatitis B in all patients. Additionally, in areas where coccidioidomycosis is prevalent, the Infectious Diseases Society of America (IDSA) strongly recommends screening for this as well.
Methods: We conducted a retrospective chart review that encompassed all patients who were on BRMs, irrespective of their medical diagnosis. The data extracted from the patient’s electronic medical records included their demographics, such as age, sex, race, and ethnicity, as well as information about the types of autoimmune diseases they had and the specific biologics used for treatment. We also looked into whether these patients underwent testing for coccidioidomycosis before commencing biologics and documented the clinical presentation of coccidioidomycosis if it was found positive during the screening process.
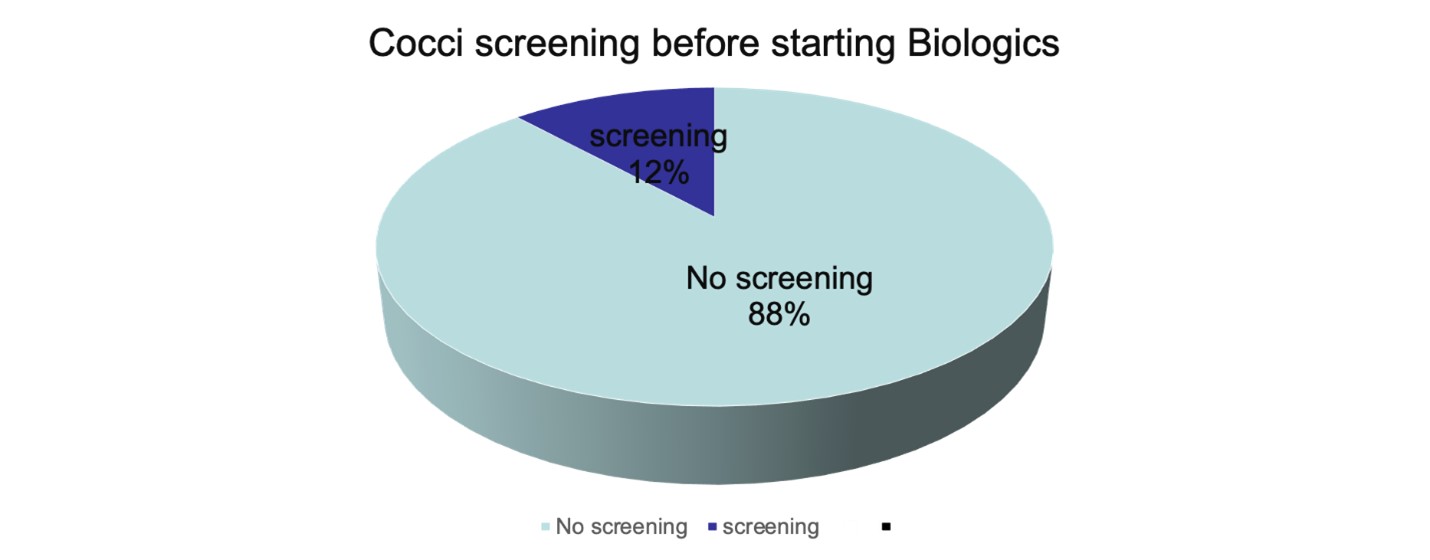
Results: A total of 255 patients who were on biologic treatment for various autoimmune conditions were identified, comprising 24 females and 231 males. The median age was recorded at 61.6. Interestingly, our findings revealed that only 12% of these patients had undergone screening for coccidiomycosis (cocci) before commencing their biologic therapy. This discovery underscores the necessity for an enhanced screening process, aiming to ensure a larger proportion of patients receive the required testing for potential cocci infection prior to initiating biologic treatment. By improving screening protocols, we can more effectively detect and manage the risk of disseminated coccidioidomycosis in patients undergoing biologic therapy, ultimately resulting in an elevation of patient safety standards.
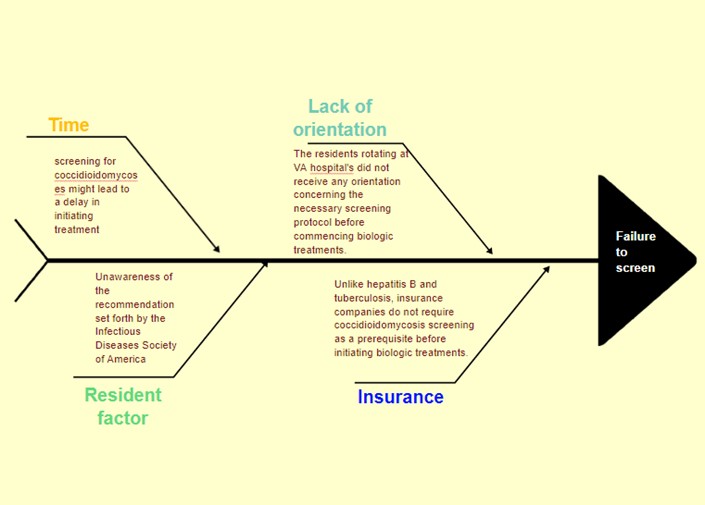
Conclusion: Based on the previous research findings and in line with the recommendations of the IDSA, our goal was to enhance the screening process for coccidioidomycosis before initiating BRMs in our hospital. After reviewing the records of 257 patients on BRMs, we found that less than 10% of them had been screened for coccidiomycosis. The main reasons for this deficiency were the lack of awareness among healthcare providers and the insurance company’s requirement for only hepatitis B screening and QuantiFERON test before approving BRM treatment.
To address this issue, we took action by educating residents about the risk of disseminated coccidiomycosis in patients on BRMs. Additionally, we have implemented an order set that includes screening for coccidioidomycosis along with QuantiFERON and hepatitis B screening to ensure comprehensive testing and improved patient safety before starting BRMs.
To cite this abstract in AMA style:
Tanous B, SAMA S, Rasheed N, Reyes C. Enhancing Pre-Biologic Response Modifier Screening for Coccidioidomycosis in Endemic Regions [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/enhancing-pre-biologic-response-modifier-screening-for-coccidioidomycosis-in-endemic-regions/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enhancing-pre-biologic-response-modifier-screening-for-coccidioidomycosis-in-endemic-regions/