Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Clinically evident rheumatoid arthritis-associated interstitial lung disease (RA-ILD) affects approximately 10-15% of patients with rheumatoid arthritis (RA) and accounts for the most overrepresented cause of death among RA patients with a reported median survival of 3 years post-diagnosis. Although the pathogenesis of RA-ILD is not well defined, common themes such as tolerance loss, autoimmunity, and tissue fibrosis are frequently implicated in contributing to disease development. Our group and others have identified potential pathogenic roles of post-translational modifications (PTMs) such as citrullination (CIT) and malondialdehyde-acetaldehyde adducts (MAA) in RA-ILD. Both anti-citrullinated protein antibodies and anti-MAA antibodies are associated with the presence of RA-ILD. Lung tissues from patients with RA-ILD exhibited intensified staining for MAA, with pronounced co-localization with CIT. Based on these findings we have hypothesized that exposure of human macrophages (MΦ) to fibrinogen (FIB) modified MAA (FIB-MAA), CIT (FIB-CIT) or MAA-CIT (FIB-MAA-CIT) leads to the secretion of soluble factors that induce robust pro-fibrotic responses in human lung fibroblasts (HLFs) in comparison to direct stimulation of HLFs with these same antigens.
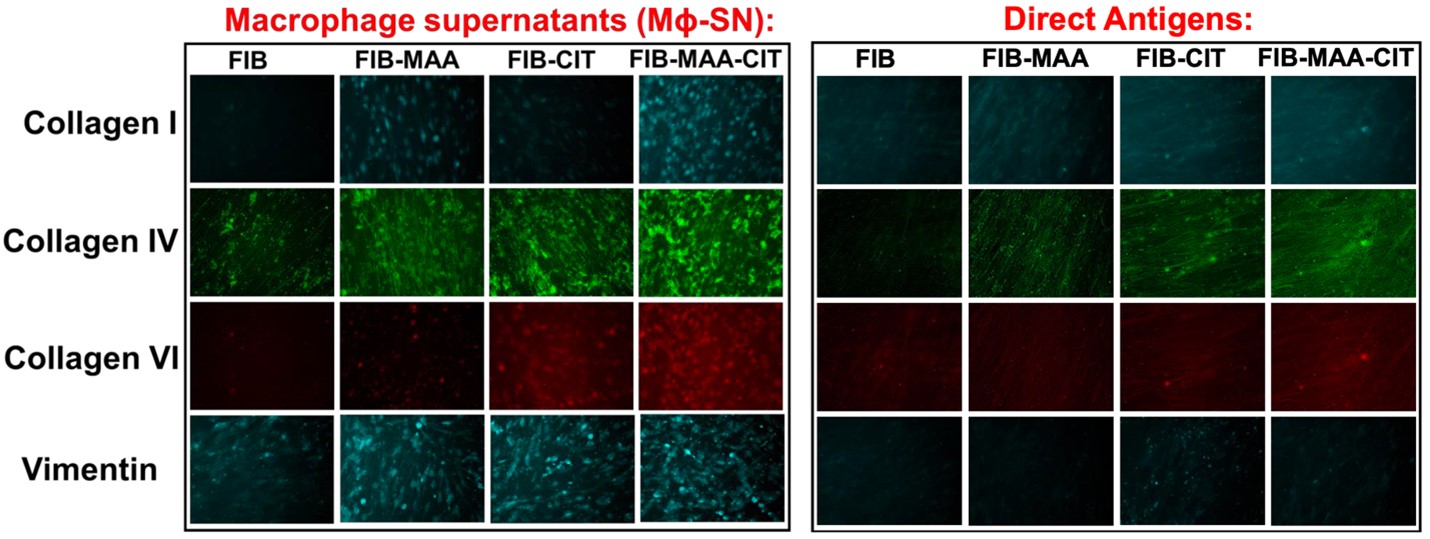
Methods: HLFs were stimulated every 48 hours for 14 days either; directly with FIB, FIB-MAA, FIB-CIT, or FIB-MAA-CIT, or indirectly with supernatants (SN) harvested from activated U937 MΦ (MΦ-SN) stimulated with the same antigens (MΦ-SNFIB-MAA, MΦ-SNFIB-CIT, and MΦ-SNFIB-MAA-CIT). Fluorescent immunohistochemistry (IHC) coupled with mean pixel density evaluations were utilized to monitor morphological changes and to quantify the levels of extracellular matrix (ECM) protein deposition including collagen I, IV, VI, and vimentin. Western blots were used to validate the findings from IHC.
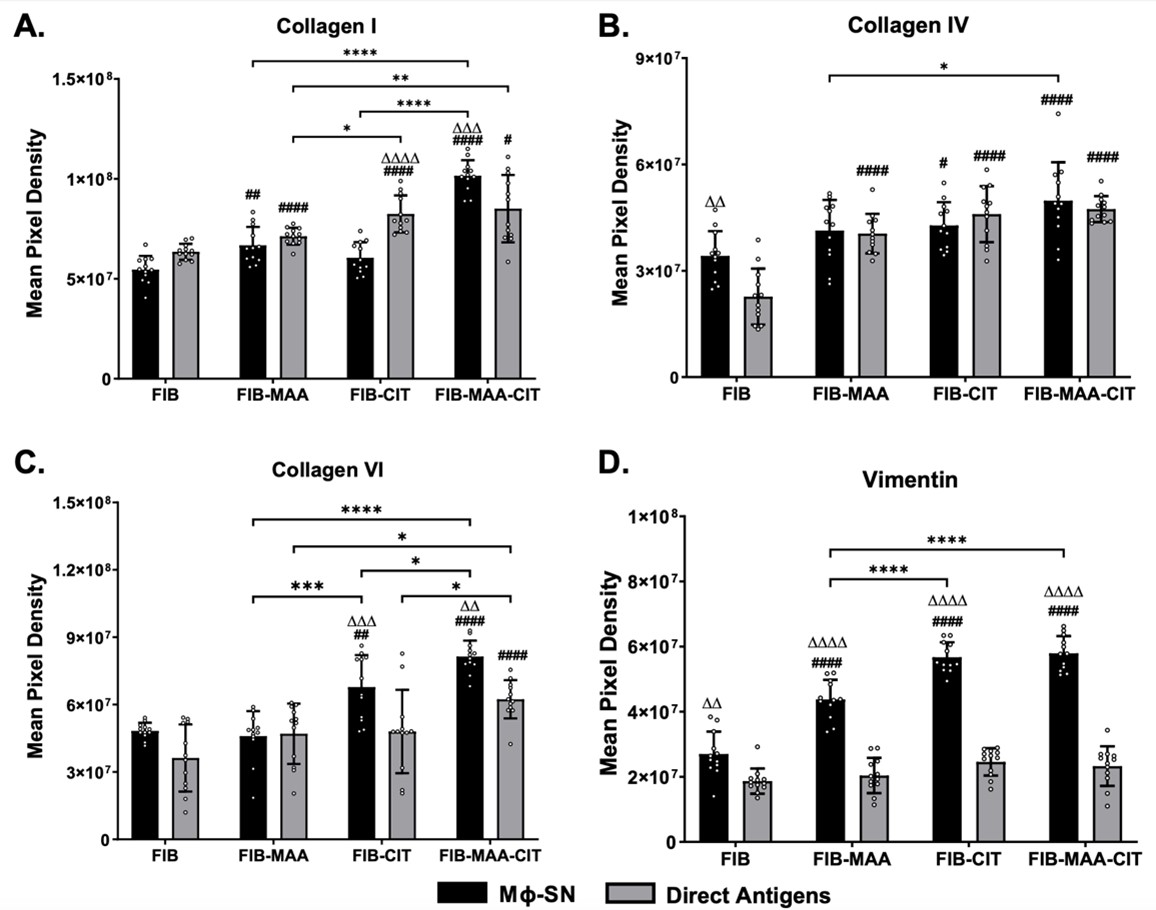
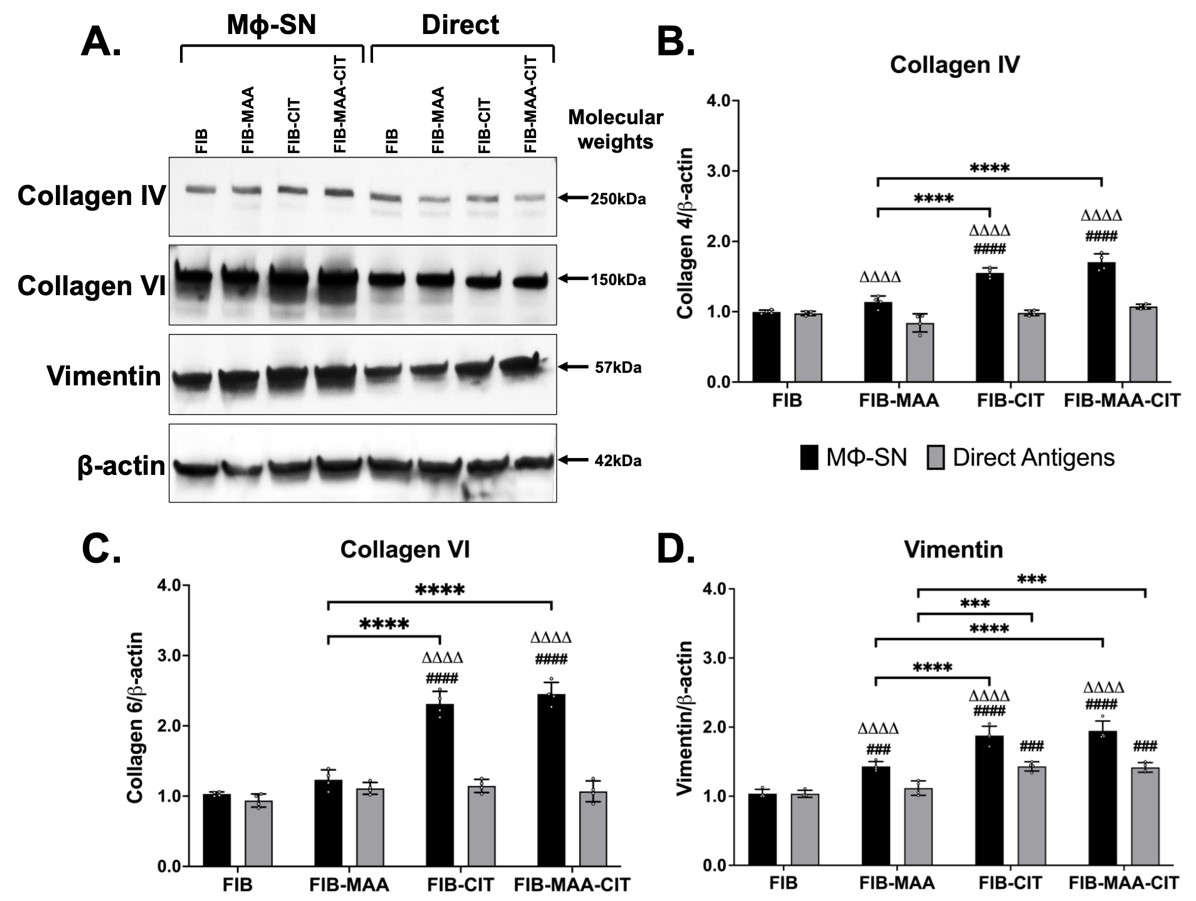
Results: Following antigenic exposure, two phenotypically distinct morphologies were observed in HLF cells. HLF cells stimulated with MΦ-SNFIB-MAA, MΦ-SNFIB-CIT, and MΦ-SNFIB-MAA-CIT demonstrated circular and spindle-shaped changes in cellular morphology consistent with cell activation (Figure 1). HLF cells stimulated with MΦ-SNFIB-MAA-CIT showed the highest deposition of collagen I and VI compared to either single modification (*p< 0.05) or direct antigen stimulation (ΔΔp< 0.01) (Figure 2). By Western Blot, HLFs treated with MΦ-SNFIB-CIT and MΦ-SNFIB-MAA-CIT demonstrated the highest expression of collagen IV, collagen VI, and vimentin compared to MΦ-SNFIB (####p< 0.001), MΦ-SNFIB-MAA (****p< 0.001) or direct antigen stimulation (ΔΔΔΔp< 0.001) (Figure 3).
Conclusion: Findings from this study demonstrate that MΦ exposed to CIT and/or MAA modified FIB secrete soluble factors that activate pro-fibrotic responses in HLF effector cells, that could potentially lead to pulmonary fibrosis associated with RA-ILD. These results suggest that interventions targeting MAA and CIT formation, or cell binding could represent a novel therapeutic approach in the management of RA-ILD.
To cite this abstract in AMA style:
Klingemann L, Aripova N, Aripova N, Duryee M, Hunter C, Poole J, England B, Mikuls T, Thiele G. Pro-Fibrotic Effects of Malondialdehyde-Acetaldehyde-Adducted and/or Citrullinated Proteins on Macrophages and Human Lung Fibroblasts [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pro-fibrotic-effects-of-malondialdehyde-acetaldehyde-adducted-and-or-citrullinated-proteins-on-macrophages-and-human-lung-fibroblasts/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pro-fibrotic-effects-of-malondialdehyde-acetaldehyde-adducted-and-or-citrullinated-proteins-on-macrophages-and-human-lung-fibroblasts/