Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: RA – Diagnosis, Manifestations, & Outcomes III: Predicting & Optimizing Outcomes
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Nintedanib and pirfenidone are antifibrotic drugs indicated for the treatment of idiopathic pulmonary fibrosis (IPF) and other forms of progressive pulmonary fibrosis. Antifibrotics have also been tested in RA-associated interstitial lung disease (ILD) within two recent randomized clinical trials (RCT). However, there are limited real-world studies regarding the use of antifibrotics, and none in RA-ILD. RA-ILD patients may be different from IPF patients since they often need concomitant immunosuppression for articular disease. Our aim was to investigate the tolerability and effectiveness of antifibrotics in a real-world cohort of patients with RA-ILD.
Methods: In this retrospective cohort study, we identified RA-ILD patients initiating antifibrotics at a large multi-hospital healthcare system. We used electronic query to find all patients with at least two RA diagnosis codes and a prescription for either nintedanib or pirfenidone (2014-2023). We then performed medical record review to confirm all patients met 2010 ACR/EULAR classification criteria for RA and had definite RA-ILD by Bongartz criteria. Data regarding adverse events (AEs), tolerability, pulmonary function test (PFT) results, and clinical data were collected. Drug retention was estimated using a Kaplan-Meier curve. A linear mixed model with random intercept was used to compare the within-patient trajectory of the percent predicted forced vital capacity (%FVC) within 18-months before to 18-months after antifibrotic initiation among those with these PFT data.
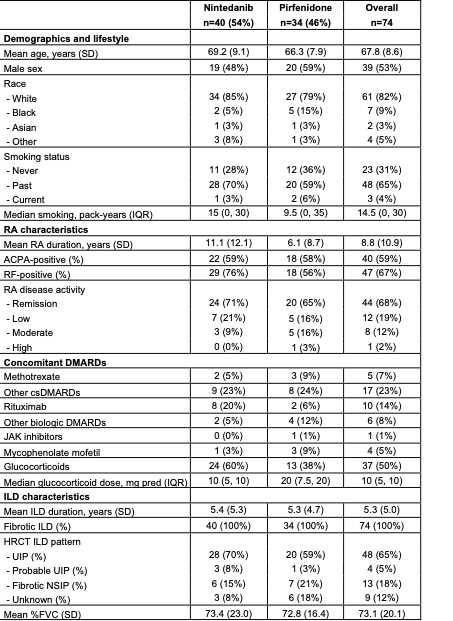
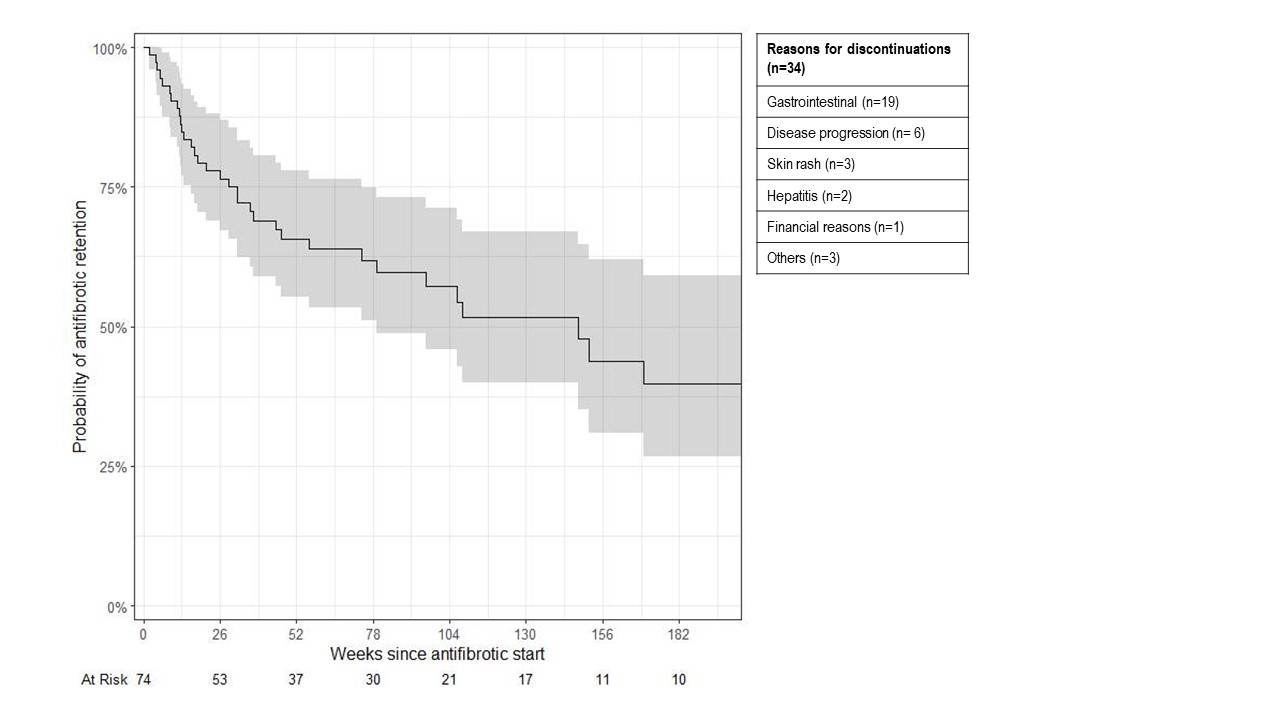
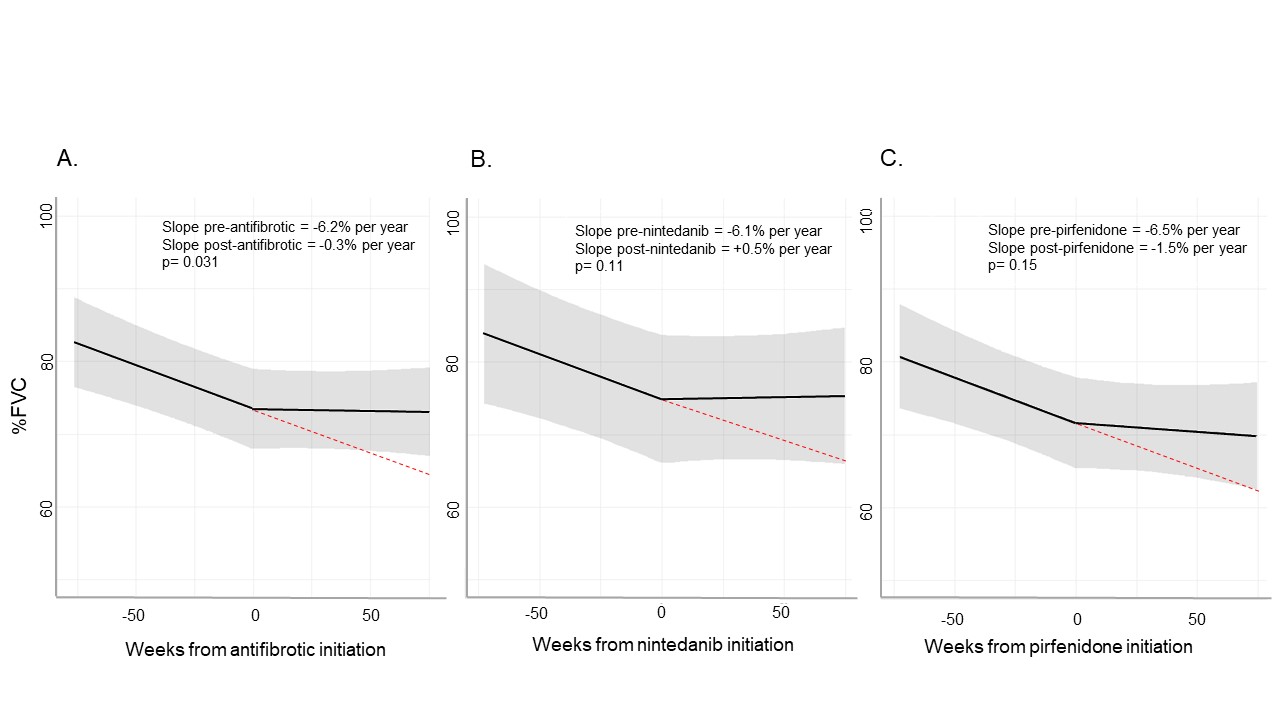
Results: We analyzed 74 patients with RA-ILD that initiated antifibrotics (mean age 67.8 years, 53% male); 40 patients initiated nintedanib and 34 initiated pirfenidone (Table 1). Median follow-up was 89 weeks (min 4, max 387). AEs were reported in 41 (55%) patients, with gastrointestinal (GI) AEs (n=30) being most common, followed by disease progression (n=6), rash (n=3), and hepatitis (n=2). The initial antifibrotic was discontinued in 34 (46%) patients due to: GI AEs (n=19), rash (n=3), transaminitis (n=2), and financial reasons (n=1). The median drug survival was 147.7 weeks (95%CI 79.1, NA; Figure 1). There was no difference in drug retention between nintedanib and pirfenidone (p=0.68). A second antifibrotic was prescribed in 14 patients, with 4 discontinuations. Change of %FVC trajectory was analyzed for 49 patients with available PFTs within 18 months pre- and post-antifibrotic (median number of PFT/patient = 4). There was a statistical difference in the estimated %FVC slope after initiation (-0.3% per year compared to -6.2% per year before initiation, p=0.031, Figure 2). Twenty-six patients (35%) died (17 due to ILD) and 4 (5%) had lung transplantation during follow-up.
Conclusion: In this first real-world study of antifibrotic use in RA-ILD, AEs were frequently reported, particularly GI, and discontinuation was common (46% compared to 20% for nintedanib and 24% for pirfenidone in their respective RCTs). However, antifibrotic initiation was associated with a modestly improved trajectory in %FVC. Death and/or lung transplant were frequent, emphasizing the need for additional safe and effective RA-ILD treatment options.
ACPA: anti-citrullinated peptides proteins, cs and bDMARDs: conventional and biologic disease modifying drugs, DLCO: diffusing lung capacity of the lungs for carbon monoxide, FVC: forced vital capacity, HRCT: high resolution computed tomography, ILD: Interstitial lung disease, NSIP: non-specific interstitial pneumonia, RA: rheumatoid arthritis, RF: rheumatoid factor, UIP: usual interstitial pneumonia.
To cite this abstract in AMA style:
Juge P, Hayashi K, McDermott G, Vanni K, Kowalski E, Qian G, Bade K, Saavedra A, Dieudé P, Dellaripa P, Doyle T, Sparks J. Tolerability and Effectiveness of Antifibrotics in Rheumatoid Arthritis-associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/tolerability-and-effectiveness-of-antifibrotics-in-rheumatoid-arthritis-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tolerability-and-effectiveness-of-antifibrotics-in-rheumatoid-arthritis-associated-interstitial-lung-disease/