Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA) was approved in 2020 in Japan for the treatment of “Rheumatoid arthritis (RA) patients with inadequate response to conventional therapy, including inhibition of structural damage progression”. All-case post-marketing Surveillance (PMS) in Japan was started at the same time of the market launch and is currently collecting data up to 3-years (24-week data for solicited events). In Japanese label, UPA15 mg should be orally administered once daily for adult RA patients. UPA 7.5 mg may be acceptable for some patients according to their condition.
Methods: This ongoing PMS includes all patients with RA prescribed with UPA since April 2020. An interim analysis of PMS was performed to evaluate the safety and the effectiveness (disease activity) of UPA for 24 weeks.
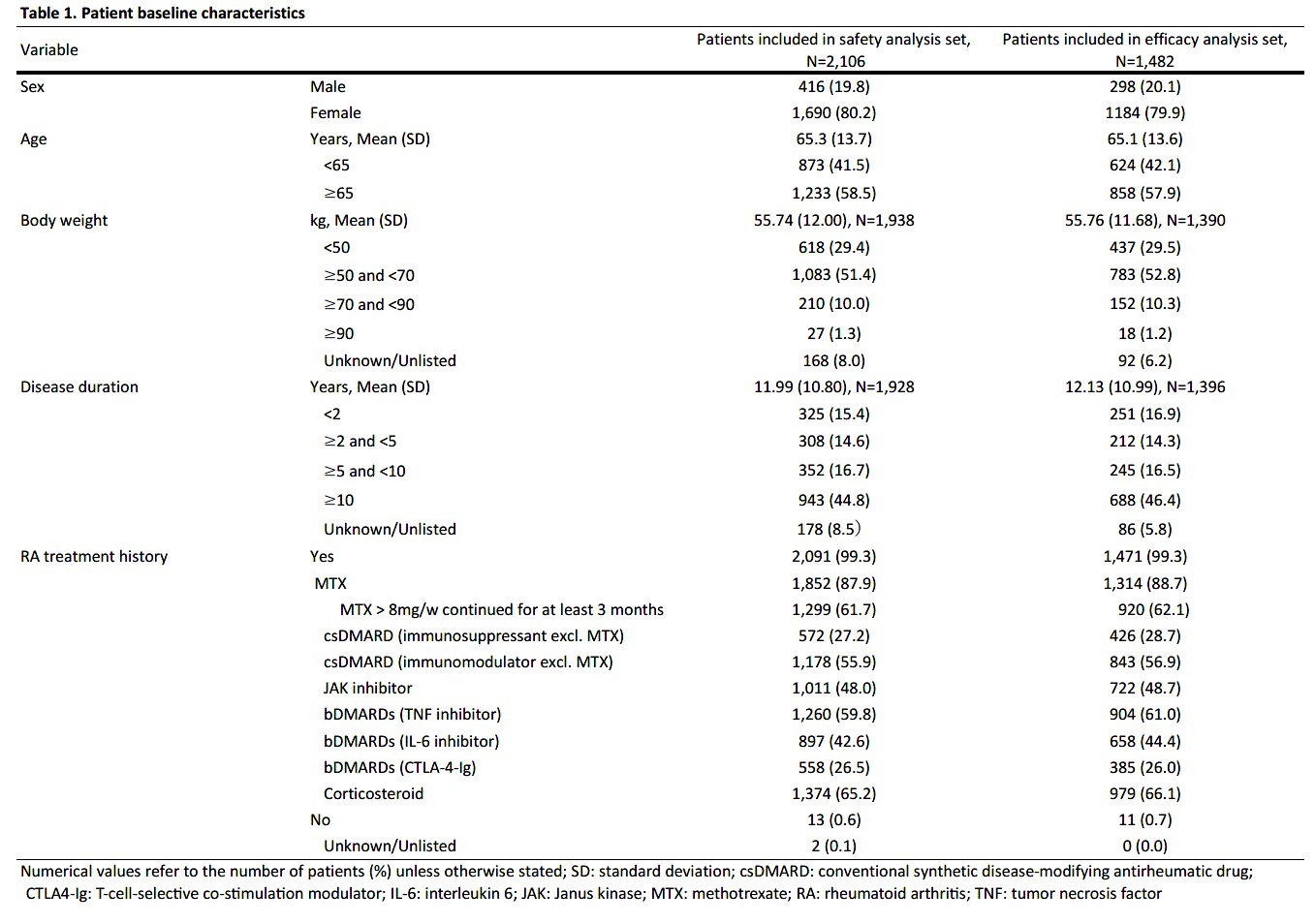
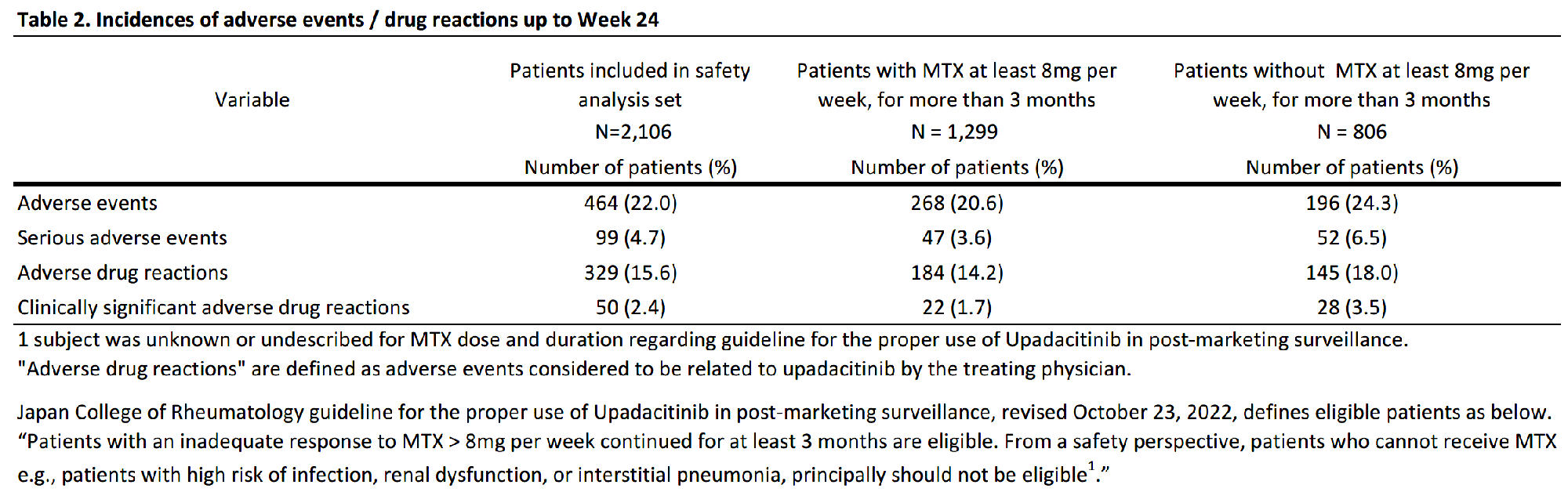
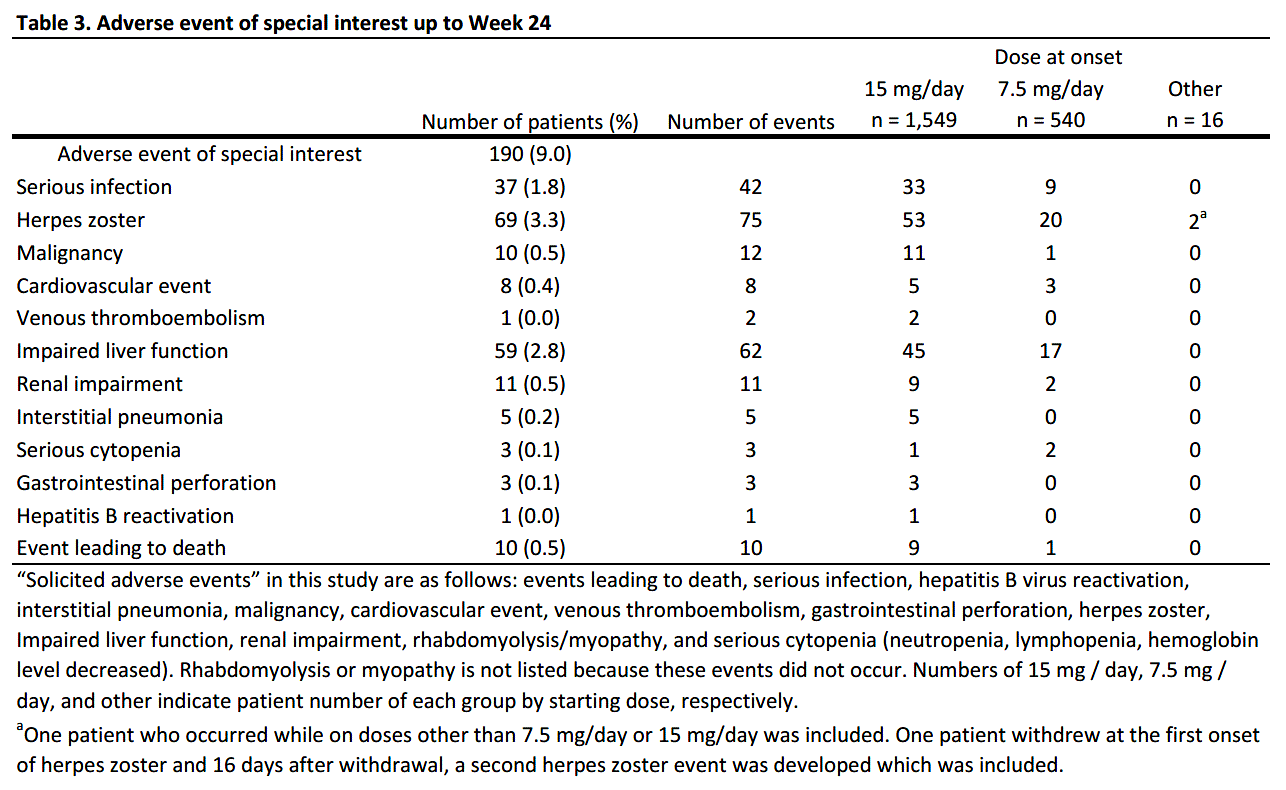
Results: As of August 15, 2022, 2878 patients were enrolled, with 2,106 and 1,482 patients included in the safety (mean age=65.3 years old; >65 years old=58.5%; female=80.2%; mean RA duration=12.0 years) and efficacy analysis set, respectively (Table 1). In the safety analysis set, 1,694 (80.4%) continued UPA treatment for 24 weeks. UPA doses at onset were 7.5mg/day in 540 (25.6%), 15mg/day in 1,549 (73.6%) of patients in the safety analysis set, respectively. At baseline, 44.4% and 41.0% of patients in the safety analysis set received methotrexate (MTX) and glucocorticoid, respectively, and 22.4% had mild or greater renal failure. Most common comorbidities were osteoporosis, hypertension, and gastroesophageal reflux disease. AEs and serious AEs (SAEs) occurred in 464 (22.0%) and 99 (4.7%) patients, respectively. In the 1,299 patients receiving MTX >8 mg/week for ≥3 months,2 AEs occurred in 268 (20.6%) and SAEs in 47 (3.6%). In the 806 patients not receiving MTX > 8mg/week for ≥3 months, AEs occurred in 196 (24.3%) and SAEs in 52 (6.5%) (Table 2). Ten deaths were reported, with causes including lung tumor, subarachnoid hemorrhage, organizing pneumonia, bacterial pneumonia, pneumocystis jirovecii, COVID-19 and unknown. Serious infections were reported in 37 (1.8%) patients. Herpes zoster in 69 (3.3%), impaired liver function in 59 (2.8%), serious cytopenia in 3 (0.1%), renal impairment in 11 (0.5%), malignancy in 10 (0.5%), cardiovascular event in 8 (0.4%), interstitial pneumonia in 5 (0.2%), and venous thromboembolism (VTE) in 1 (0.0%) were reported, respectively (Table 3). 6 of 10 patients with malignancies were diagnosed within 2 months of UPA initiation. 621 patients were assessed in DAS28-CRP, of which 488 (78.6%) achieved LDA or CR and 382 (61.5%) achieved CR.
Conclusion: The 24-week interim analysis of all-case PMS in Japan showed that more than half of the patients receiving UPA are over aged 65 years or older. Although the mean age is higher than that of patients in clinical studies, the safety profile was consistent with clinical trials, and no new safety signals were identified.3,4 The safety and efficacy data from this real-world setting will continue to be collected to assess for RA patients treated with UPA in Japan.
References
1 Mod Rheumatol suppl 2023(33): S179, presented at the JCR2023, Apr 24–26, Japan.
2 JCR guideline for proper use of UPA in PMS
3 Burmester, et al. RMD Open 2023;9: e002735
4 Yamaoka, et al. Drug Saf. 2021 Jun;44(6):711-722.
To cite this abstract in AMA style:
Atsumi T, Okamoto N, Takahashi N, Tamura N, Nakajima A, Nakajima A, Fujii T, Matsuno H, Kawaberi T, Sunaga N, Tsujita Y, Chonan S, Kuwana M, Takagi M. 24-week, Post-Marketing Surveillance Analysis of Upadacitinib in Japanese Patients with Rheumatoid Arthritis (Encore1) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/24-week-post-marketing-surveillance-analysis-of-upadacitinib-in-japanese-patients-with-rheumatoid-arthritis-encore1/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/24-week-post-marketing-surveillance-analysis-of-upadacitinib-in-japanese-patients-with-rheumatoid-arthritis-encore1/